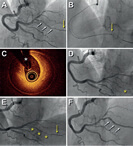
A 59-year old male with severe left ventricular dysfunction, slow ventricular tachycardia and previously treated with cardiac resynchronization therapy was referred for percutaneous coronary intervention (PCI) of the left anterior descending coronary artery (LAD). The patient had 2 severely calcified lesions at mid-LAD level. Because it was a high-risk PCI, the heart team decided to perform this procedure using percutaneous left ventricular support using the Impella CP device (Abiomed, United States). The patient had a past medical history of right femoral-popliteal bypass surgery and a severely calcified stenosis in his left external iliac artery (figure 1A, arrow). The computed tomography performed on the supraaortic arteries revealed subclavian and axillary arteries with diameters < 5mm (figure 1B). The Impella CP device (14-Fr)—that requires a minimal vessel diameter of 5mm—was implanted using the left femoral access after revascularization of left external iliac artery. Predilation was performed using a 5 x 40 mm Mustang PTA balloon catheter (Boston Scientific, United States) (figure 1C); afterwards a 6 x 38 mm balloon expandable covered stent Advanta V12 (Atrium Medical Corporation, United States) was implanted (figure 1D,E). The Impella CP device was advanced through the stent (figure 1F). The PCI of the mid-LAD was performed using 2 drug-eluting stents (figure 1G,H; arrows: lesions at mid-LAD level). After the PCI, the Impella CP device was retrieved; vascular access was closed using Perclose ProGlide (Abbott Vascular Inc., United States). The control angiography showed good results (figure 1, video 1 of the supplementary data).
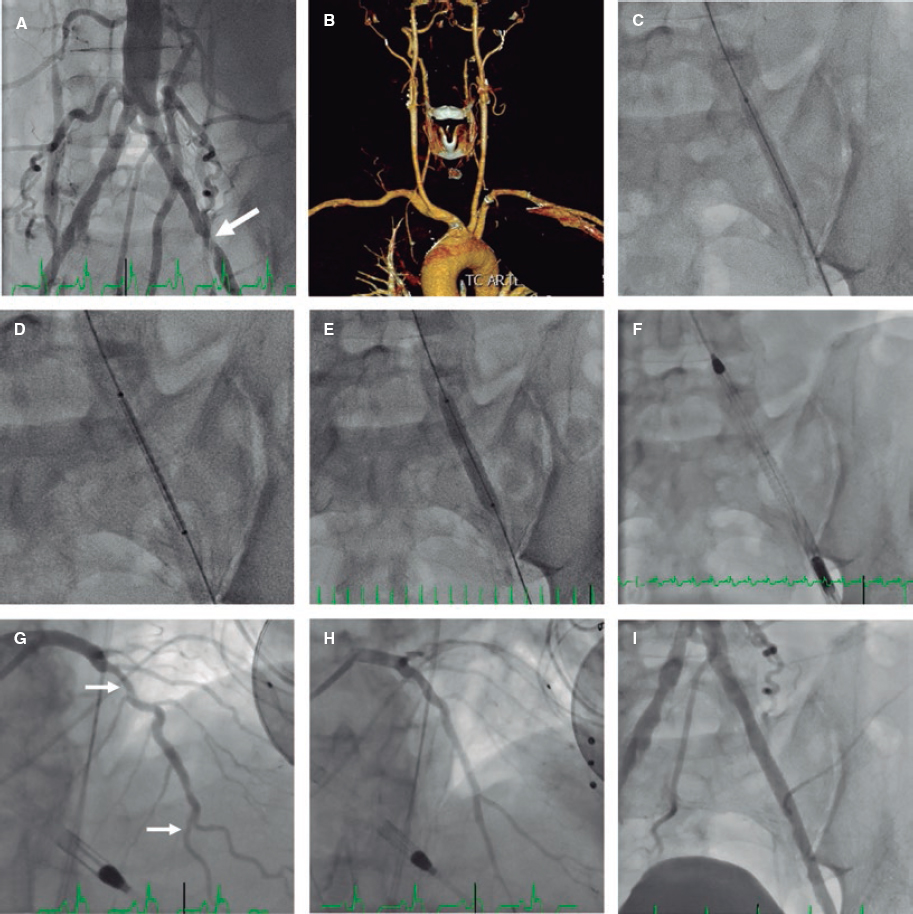
Figure 1.
The percutaneous revascularization of the iliac artery with a covered stent may be an alternative approach to advance the Impella CP device and facilitate high-risk PCIs when subclavian and axillary accesses are not an option for having small diameters.
SUPPLEMENTARY DATA
Video 1. Cubero-Gallego H. DOI: 10.24875/RECICE.M20000097
Corresponding author: Área del Corazón, Hospital Universitario Central de Asturias, Avda. Roma s/n, 33011 Oviedo, Asturias, Spain.
E-mail address: hektorkubero@hotmail.com (H. Cubero-Gallego).
The calcified nodule has been established as the least common underlying substrate in the acute coronary syndrome; however calcium can be widely present in other acute-common scenarios. In this setting, the optical coherence tomography (OCT) study has identified 3 different types of calcification according to morphology. Former studies have already documented calcified nodules in patients with peripheral artery disease; however the different patterns that exist beyond coronary arteries remain unknown.
An 80-year-old patient was referred for coronary angiography following systolic dysfunction. The coronary angiography performed through right radial approach showed a severely calcified vessel (mid portion of the left anterior descending artery) and mitral and aortic calcification. The OCT pullback performed with 6 mL of contrast at 3 mm/seg through the radial artery removed 4 cm of the radial sheath and showed multiple calcified plaques (mid portion of the artery) defined by the presence of superficial, well-established, low-backscattering and heterogeneous regions (video 1 of the supplementary data). The simultaneous angiography co-registration confirmed the presence of ulnar artery occlusion. Three patterns of superficial calcification were identified:
- – Calcified protrusion (protruding calcified mass without eruptive nodules [figure 1A,B]).
- – Eruptive calcified nodules (cluster of small calcified nodules protruding into the lumen [figure 1C,D]).
- – Sheet-like superficial calcified plate (without superficial coating disruption and minimal laminar protrusion [figure 1E,F]).
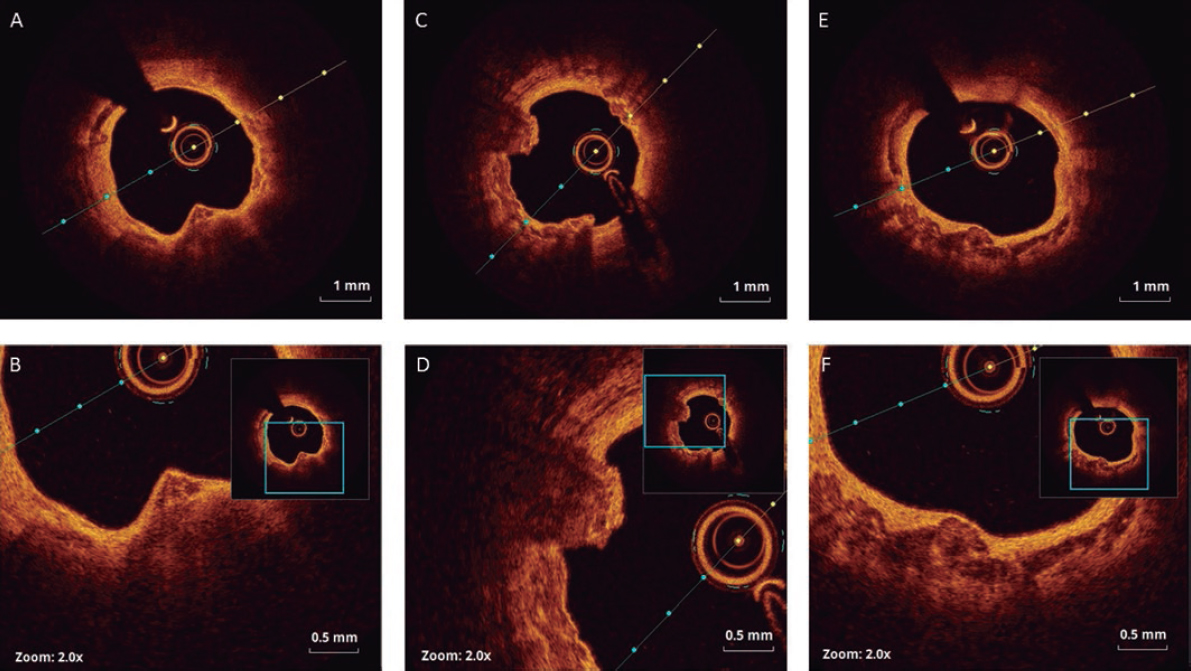
Figure 1.
The types and causes of peripheral calcification described included calcified atherosclerosis, calcific medial vasculopathy, elastocalcinosis, and calcific uremic arteriolopathy. This is the first time that an OCT study describes calcification patterns of atherosclerotic plaques in peripheral artery disease. As previously defined for calcified coronary culprit lesions, 3 types could be identified. The coexistence of ulnar artery occlusion suggests causality, but further studies will be needed to clarify its pathological meaning in the setting of the acute peripheral syndrome.
CONFLICTS OF INTEREST
R. Moreno Gómez is associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
SUPPLEMENTARY DATA
Video 1. Arroyo-Úcar E. DOI: 10.24875/RECICE.M20000094
Corresponding author: Servicio de Cardiología, Hospital de Vinalopó, Tonico Sansano Mora 14, 03293 Elche, Alicante, Spain.
E-mail address: earroyoucar@gmail.com (E. Arroyo-Úcar).
Although the most common cause for coronary embolism is atrial fibrillation, we should take other conditions into consideration that, despite their low frequency of occurrence, need to be discarded to be able to establish a definitive treatment. Arteriovenous malformations like the case presented here are some of these conditions.
A 36-year-old woman with Rendu-Osler-Weber syndrome and recurrent and spontaneous epistaxis as the only personal medical history presented to the ER with oppression in her middle chest and pain radiating towards her left upper limb and back with concomitant vegetative symptoms. The electrocardiogram confirmed the presence of a subepicardial lesion in leads V2-V3 with high-sensitive troponin peak values of 9148 pg/mL.
A coronary angiography performed early confirmed the presence of a thrombus at the circumflex artery distal bifurcation (figure 1, arrow).
Figure 1.
To discard emboligenic source a Doppler ultrasound scan of the lower limbs was performed that revealed no signs of deep venous thrombosis. Given the patient’s syndromic history, a thoracic computed tomography angiography was performed to look for pulmonary arteriovenous fistulas and found 2 of them: one located at the left pulmonary territory in the posterior basal segment (figure 2, arrow) and another smaller one in the homolateral lateral basal segment.
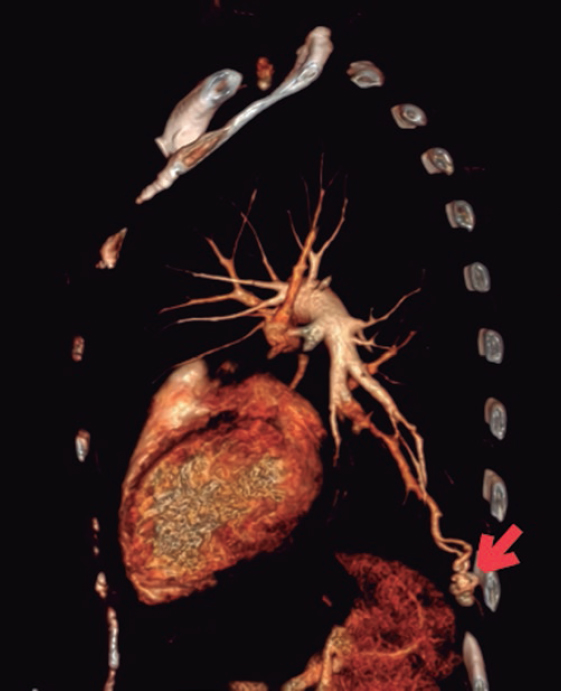
Figure 2.
The interventional cardiovascular unit used selective microcatheterization of the segmental arteries afferent to the fistulas and coil embolization to close them (figure 3, arrows).
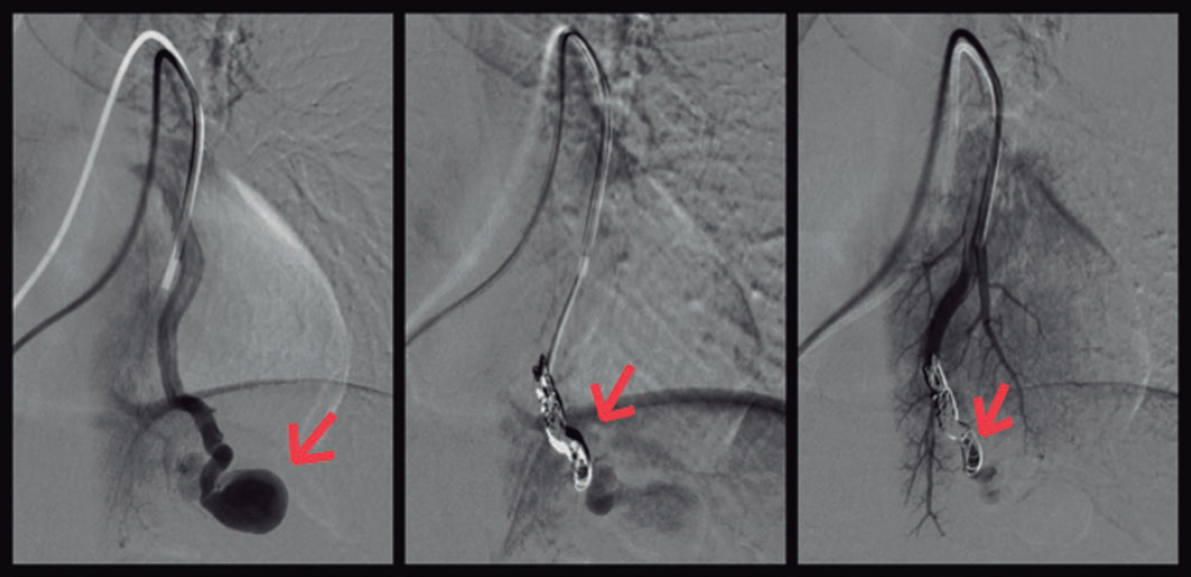
Figure 3.
Corresponding author: Unidad de Cuidados Intensivos Cardiológicos, Hospital Universitario Clínico San Cecilio, Avda. del Conocimiento s/n, 18016 Granada, Spain.
E-mail address: antoniocarranzapinel.ac@gmail.com (A. Carranza Pinel).
A 48-year-old woman with congenital pulmonary stenosis who required surgical valvuloplasty in 1978 presented with progressive dyspnea. The cardiovascular magnetic resonance imaging performed confirmed the presence of dilated right ventricle, severe regurgitation, and pulmonary artery aneurysm (39 × 25 mm). The heart team decided to perform a transcatheter pulmonary valve implantation. During pre-stenting with an uncovered 15-25 mm × 47-55 mm CP Stent (NuMED, United States) mounted on a 25 mm balloon of the native right ventricular outflow tract, stent embolization with spontaneous anchoring to the left pulmonary artery occurred (video 1 of the supplementary data, and figure 1A). Since the patient remained stable, a wait-and-see approach was decided to facilitate stent endothelialization. The stent (figure 1B) was used as the anchoring substrate 2 months apart of the proximal implantation for 2 longer Andrastent XXL 57 mm-stents (Andramed, Germany) on a 30 × 40 mm XL AndraBalloon to create a landing zone for the 29 mm Sapien-3 valve. The rest of the procedure was successful (figure 1C). The patient remained asymptomatic, with no perfusion defects as confirmed by the ventilation/perfusion lung scan and a mean transvalvular gradient of 7 mmHg without any residual regurgitation at the 6-month follow-up (figure 1D).
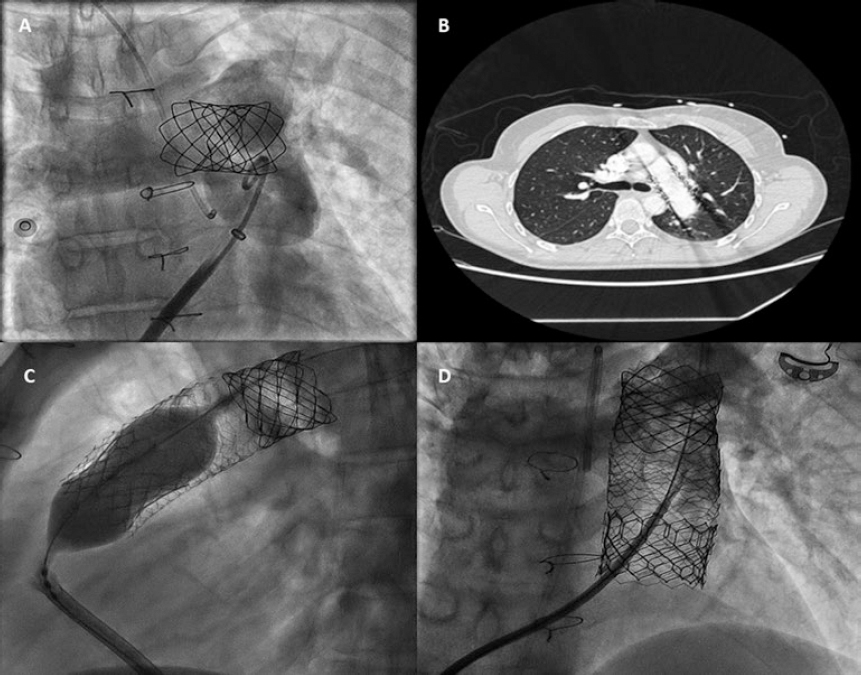
Figure 1.
In cases of aneurysmal pulmonary trunk and dilated native/non-calcified right ventricular outflow tract, the high risk of stent or valve migration may be prevented by the “planned” implantation of a first stent of smaller dimensions in a pulmonary branch. Then, sequential proximal stents may be anchored to this landing zone, which facilitates the reconstruction of pulmonary trunk with low risk of flow compromise in the jailed pulmonary branch. Further studies to assess this scenario are warranted.
CONFLICTS OF INTEREST
J.L. Zunzunegui is a proctor for Edwards Lifesciences.
SUPPLEMENTARY DATA
Video 1. Aparisi A. DOI: 10.24875/RECICE.M19000088
Corresponding author: Instituto de Ciencias del Corazón (ICICOR), Hospital Clínico Universitario de Valladolid, Ramón y Cajal 3, 47005 Valladolid, Spain.
E-mail address: ijamat@gmail.com (I.J. Amat-Santos).
A 41-year-old woman presented with an inferolateral ST-segment elevation myocardial infarction. Coronary angiography confirmed the presence of a long narrowing involving the right coronary artery posterolateral branch (figure 1A, white arrows). Spontaneous coronary artery dissection was suspected and optical coherence tomography (OCT) imaging was considered by the operator for confirmation purposes. Advancing the OCT catheter was difficult due to lack of support and it was decided to only interrogate the proximal aspect of the branch (figure 1B, arrow). OCT revealed the presence of an intramural hematoma without entry tear (figure 1C; asterisks denote wire artefact). At that moment the patient complained of chest pain that remained after removing the OCT catheter. The presence of functional branch occlusion (figure 1D, asterisk), with Thrombolysis in Myocardial Infarction (TIMI) grade 1 flow, was confirmed. Coronary flow was re-established through gentle dilation (4 atm) using a 1.5 mm balloon. Final TIMI grade 2 flow was achieved (figure 1E, asterisks) and the patient became asymptomatic. Her subsequent clinical course was uneventful. Coronary angiography performed 3 months later confirmed the complete healing of the dissected segment (figure 1F, arrows).
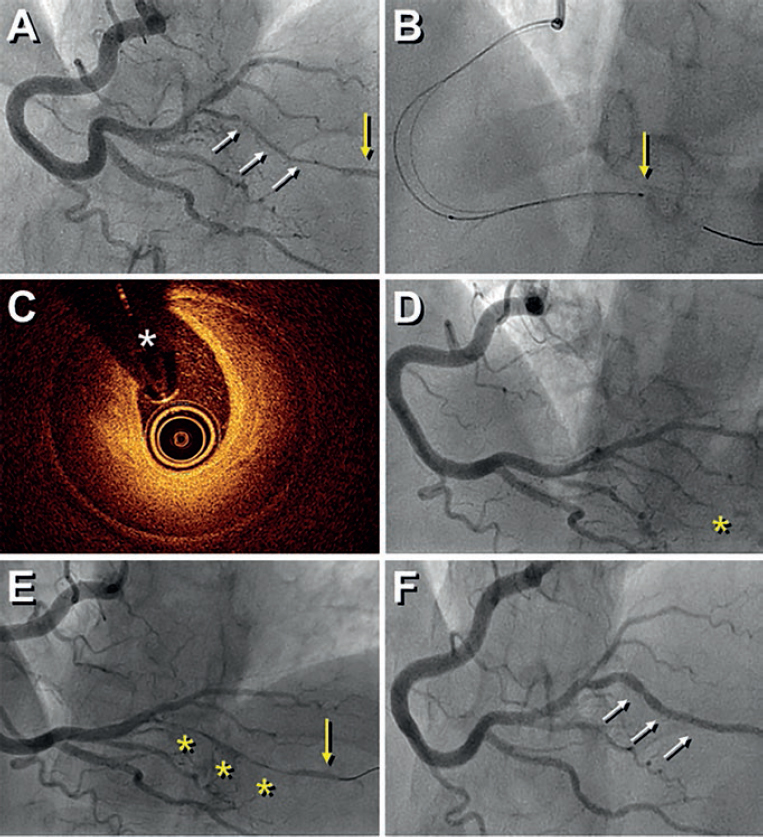
Figure 1.
Diagnosing spontaneous coronary artery dissection can be challenging in the absence of the classical dual-lumen pattern. OCT imaging is considered safe and, therefore, recommended for selected patients to confirm the diagnosis. Our findings illustrate that extra care is required while acquiring images in these frail and disrupted vessels. OCT should only be considered when the diagnosis remains unclear, and the acquisition of images in very distal and small vessels should be avoided.
CONFLICTS OF INTEREST
F. Alfonso is an associate editor of
Corresponding author: Departamento de Cardiología, Hospital Universitario de La Princesa, Diego de León 62, 28006 Madrid 28006, Spain.
E-mail address: falf@hotmail.com (F. Alfonso).
Editorials
Transcatheter aortic valve replacement for noncalcified aortic regurgitation. Where are we now?
aServicio de Cardiología, Hospital Clínico Universitario, Valladolid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain
Original articles
Editorials
Vascular closure devices: the jury is still out
aUnidad de Hemodinámica, Servicio de Cardiología, Hospital General Universitario Dr. Balmis, Instituto de Investigación Sanitaria y Biomédica de Alicante (ISABIAL), Alicante, Spain
bDepartamento de Medicina Clínica, Universidad Miguel Hernández, Alicante, Spain
Original articles
Debate
Debate: Asymptomatic severe aortic stenosis: when should we intervene?
The clinician’s perspective
Servicio de Cardiología, Hospital Ramón y Cajal, Madrid, Spain
The interventional cardiologist’s perspective
Unidad de Cardiología Intervencionista, Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo, Vigo, Pontevedra, Spain