ABSTRACT
Introduction and objectives: Contrast-induced-acute kidney injury (CI-AKI) is a potential complication of angiographic procedures. The DyeVert Contrast Reduction system (Osprey Medical, United States) is a device to reduce the concentration of contrast medium (CM) in the kidneys by decreasing the amount of CM delivered to patients. Unlike manual systems, few data are available on the DyeVert Power XT system, which is used in conjunction with automated contrast injection. The main aim of our study was to evaluate its effectiveness during percutaneous coronary interventions (PCI).
Methods: Between 2020 and 2022, 101 patients who underwent PCI with the DyeVert Power XT system (case group) were enrolled to evaluate the amount of CM saved through the use of this device, as well as the rate, severity, and predictors of CI-AKI. Patients who underwent PCI without the use of the device (control group) were enrolled to create a matched group allowing assessment of differences in CM and the CI-AKI rate.
Results: : In the case group, the amount of CM saved was 114 ± 42 mL, representing an average of 32% of the total CM. Fourteen patients (13.9%) developed CI-AKI. The only independent predictors of CI-AKI were hematocrit (OR, 0.86; 95%CI, 0.74-0.99; P = .04) and ejection fraction (OR, 0.88; 95%CI, 0.82-0.95; P = .001). As a result of diversion by the device, the amount of CM delivered was lower in the case group than in controls (252 vs 267 mL; P = .42), but this difference was nonsignificant. Equally, the reduction in CI-AKI (14.3% vs 16.3%) was nonsignificant.
Conclusions: Hematocrit and ejection fraction may be more important predictors of CI-AKI than the CM volume normally used during PCI in the general population. The net practical benefit of DyeVert Power XT was low.
Keywords: Acute kidney injury. Contrast media. Percutaneous coronary intervention. DyeVert.
RESUMEN
Introducción y objetivos: La nefropatía inducida por contraste (NIC) es una potencial complicación de los procedimientos angiográficos. El sistema DyeVert Power (Osprey Medical, Estados Unidos) permite reducir la concentración renal del medio de contraste al disminuir la cantidad administrada a los pacientes. Al contrario que sobre los sistemas manuales, existen pocos datos disponibles sobre el sistema DyeVert, que se utiliza junto a la inyección automática de contraste. El objetivo principal de este estudio fue evaluar su eficacia en procedimientos de intervencionismo coronario percutáneo (ICP).
Métodos: Entre 2020 y 2022 se incluyó a 101 pacientes a quienes se realizó ICP utilizando el sistema DyeVert Power XT (grupo de casos) para evaluar la cantidad ahorrada de medio de contraste, así como la tasa, la gravedad y los predictores de NIC. Además, se seleccionó un grupo control de pacientes a los que se había realizado ICP sin utilizar el sistema DyeVert para comparar la cantidad de medio de contraste administrado y la tasa de NIC.
Resultados: En el grupo de casos se redujo la administración de medio de contraste en 114 ± 42 ml (una media del 32% del total). Desarrollaron NIC 14 pacientes (13,9%). Los predictores de NIC fueron el hematocrito (OR = 0,86; IC95%: 0,74-0,99; p = 0,04) y la fracción de eyección (OR = 0,88; IC95%: 0,82-0,95; p = 0,001). Como resultado de la utilización del sistema DyeVert, la cantidad administrada de medio de contraste fue menor, pero sin diferencias estadísticamente significativas (252 frente a 267 ml; p = 0,42). La tasa de NIC fue menor con el sistema DyeVert, pero sin alcanzar la significación estadística (14,3 frente a 16,3%; p = 1,0).
Conclusiones: El hematocrito y la fracción de eyección, más que la cantidad de contraste administrada, pueden ser predictores de NIC en los pacientes que reciben ICP. El beneficio del sistema DyeVert fue bajo.
Palabras clave: Insuficiencia renal aguda. Medios de contraste. Intervención coronaria percutánea. DyeVert.
Abbreviations CI-AKI: contrast induced-acute kidney injury. CM/CMV: contrast medium/contrast medium volume. PCI: percutaneous coronary intervention.
INTRODUCTION
Contrast induced-acute kidney injury (CI-AKI) is a dreaded complication after diagnostic and interventional angiographic procedures and is linked to increased morbidity and mortality. In a large recent meta-analysis, the pooled incidence of CI-AKI after coronary angiography was 12.8%, with 95% confidence interval (95%CI) 11.7%-13.9%, and the associated mortality was 20.2% (95%CI, 10.7%-29.7%).1 Multiple risk factors have been identified: contrast medium volume (CMV), advanced age (> 75 years), diabetes, anemia, conditions associated with hypotension, and ejection fraction (EF) < 40%.2,3 Many of these risk factors are included in the Mehran score,2 which identifies 4 risk classes of contrast-induced nephropathy (CIN) after PCI: low (≤ 5 points), moderate (6-10 points), high (11-15 points), and very high (≥ 16 points). The Mehran score and the recent Mehran 2 score4 assign 1 point for each 100 mL of CMV up to a dose of 299 mL. Because volume depletion increases the CM concentration in renal tubules, the main preprocedural measure to reduce the occurrence of CI-AKI is intravenous administration of normal saline before and after the procedure, because other solutions provide no benefits5; hydration should be started 12 hours before and continued for 24 hours after the procedure at 1 mL/kg/h or 0.5 mL/kg/h if EF < 35% or New York Heart Association (NYHA) class > 2.6 Another means of decreasing CM concentration in the kidneys is the DyeVert Contrast Reduction system (Osprey Medical Inc, United States), which reduces the amount of CMV delivered to patients during angiographic procedures, with noninferior image quality as attested by independent reviewers.7,8 The DyeVert, DyeVert Plus and DyeVert Plus EZ are used in conjunction with manual contrast injection, and the DyeVert Power XT is used with automated contrast injection; the latter system has been little studied. The main aim of our study was to evaluate the effectiveness of the DyeVert Power XT system in reducing CM delivery during PCI.
METHODS
Study population
This single center, observational study was performed in patients who underwent PCI between September 2020 and December 2022 with the DyeVert Power XT system (case group) and in patients who underwent PCI during a similar period without the use of the device (control group).
Inclusion criteria for both groups were as follows: chronic kidney disease (CKD) [estimated glomerular filtration rate (eGFR) < 60 mL/min/m2] and/or need for a complex PCI with the likelihood of receiving a large amount of CM; previous coronary artery bypass graft (CABG); chronic total occlusion (CTO) (complete blockage of a coronary artery lasting at least 3 months); bifurcation; and left main and/or multivessel disease (at least 2 vessels involved).
The exclusion criterion for both groups was the presence of end-stage kidney failure on dialysis treatment. We collected laboratory, instrumental, clinical, and procedural variables in the case and control groups. Definitions of all these variables are reported in table 1, table 2, table 3, and table 4. For the variables included in the Mehran score, we used the same descriptions as those used in the score. eGFR was calculated by the Modification of Diet in Renal Disease (MDRD) 4-variable equation, left ventricular EF by 2-dimensional echocardiography during hospitalization and before arrival in the catheterization laboratory, and the risk of any post-PCI CIN by the Mehran score. Bifurcation/left main treatment (with single/double stent) consisted of the proximal optimization technique (POT) with kissing balloon inflation and eventually re-POT in all cases. Total CMV represents the volume that would have been delivered if DyeVert had not been used, ie, the sum of CMV delivered to patients and the CMV saved by DyeVert. CM injection flow was 4 and 3 mL/sec for the left and right coronary artery, respectively.
Table 1. Laboratory, instrumental, clinical characteristics, and Mehran score in the overall population and according to incidence of CI-AKI in the case group
| Characteristics | Overall population (n = 101) | No CI-AKI (n = 87) | CI-AKI (n = 14) | P |
|---|---|---|---|---|
| Laboratory and istrumental characteristics | ||||
| eGFR, mL/min | 51 ± 18 | 52 ± 19 | 45 ± 16 | .18 |
| HCT | 38.6 ± 4.9 | 39.1 ± 4.8 | 35.5 ± 4.8 | .01* |
| EF | 50 [35-55] | 50 [40-55] | 30 [28-36] | < .001* |
| CKD [eGFR < 60 mL/min/ 1.73 m2] | 73 (72.3) | 63 (72.4) | 10 (71.4) | 1 |
| Anemia [male HCT < 39%, female HCT < 36%] | 48 (47.5) | 38 (43.7) | 10 (71.4) | .10 |
| Clinical characteristics | ||||
| Age, years | 74 (68-80) | 73 (67-80) | 75 (74-81) | .09 |
| Age > 75 years | 39 (38.6) | 32 (36.8) | 7 (50) | .52 |
| Male sex | 80 (79.2) | 68 (78.2) | 12 (85.7) | .73 |
| Overweight [body mass index ≥ 25] | 52 (51.5) | 46 (52.9) | 6 (42.9) | .68 |
| Hypertension | 78 (77.2) | 70 (80.5) | 8 (57.1) | .08 |
| Diabetes | 48 (47.5) | 40 (46) | 8 (57.1) | .62 |
| Dyslipidemia | 68 (67.3) | 57 (66) | 11 (79) | .51 |
| Current smoker | 24 (23.8) | 20 (23) | 4 (28.6) | .74 |
| Former smoker | 35 (34.7) | 32 (36.8) | 3 (21.4) | .37 |
| CHF [NYHA class ≥ 3 and/or history of pulmonary edema] | 37 (36.6) | 25 (28.7) | 12 (85.7) | < .001* |
| Acute coronary syndrome presentation | 38 (37.6) | 31 (35.6) | 7 (50) | .46 |
| Hypotension [Systolic arterial pressure < 80 mmHg for ≥ 1 h requiring inotrope] | 4 (4) | 2 (2.3) | 2 (14.3) | .09 |
| Mehran score | ||||
| Mehran CI-AKI risk class: | ||||
| Low | 24 (23.8) | 24 (27.6) | 0 (0) | .04* |
| Moderate | 26 (25.7) | 24 (27.6) | 2 (14.3) | .51 |
| High | 34 (33.7) | 29 (33.3) | 5 (35.7) | 1 |
| Very high | 17 (16.8) | 10 (11.5) | 7 (50) | .002* |
| Mehran score, points | 11 ± 5 | 10 ± 5 | 15 ± 4 | < .001* |
Values are expressed as No. (%), mean ± standard deviation, or median [first quartile-third quartile]. *Statistically significant P-value (P < .05). CHF, congestive heart failure; CI-AKI, contrast induced-acute kidney injury; CKD, chronic kidney disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HCT, hematocrit; NYHA, New York Heart Association. | ||||
Table 2. Procedural characteristics in the overall population and according to incidence of CI-AKI in the case group
| Characteristics | Overall population (n = 101) | No CI-AKI (n = 87) | CI-AKI (n = 14) | P |
|---|---|---|---|---|
| Procedural characteristics (angiography/PCI complexity/complications) | ||||
| Previous CABG | 20 (19.8) | 18 (20.7) | 2 (14.3) | .73 |
| CTO [complete blockage of a coronary artery lasting at least 3 months] | 12 (11.9) | 11 (12.6) | 1 (7.1) | 1 |
| No. vessels treated in the same procedure: | ||||
| 1 | 57 (56.4) | 52 (59.8) | 5 (35.7) | .09 |
| 2 | 40 (39.6) | 32 (36.8) | 8 (57.1) | .15 |
| 3 | 4 (4) | 3 (3.4) | 1 (7.1) | .45 |
| No. bifurcations treated in the same procedure: | ||||
| 0 | 67 (66.3) | 58 (66.7) | 9 (64.3) | 1 |
| 1 | 31 (30.7) | 27 (31) | 4 (28.6) | 1 |
| 2 | 3 (3) | 2 (2.3) | 1 (7.1) | .36 |
| Left main treatment | 25 (24.8) | 20 (23) | 5 (35.7) | .33 |
| Stent, number | 2 [1-3] | 2 [1-3] | 2 [1-3] | .75 |
| Stent lenght, mm | 52 [31-88] | 51 [30-91] | 57 [36-73] | .95 |
| Perforation | 3 (3) | 3 (3.4) | 0 (0) | 1 |
| IABP use | 1 (1) | 0 (0) | 1 (7.1) | .14 |
| Rotablator use | 3 (3) | 1 (1.1) | 2 (14.3) | .05 |
| Procedural characteristics (others) | ||||
| Radial access | 88 (87.1) | 75 (86.2) | 13 (92.9) | .69 |
| Femoral access | 27 (26.7) | 21 (24.1) | 6 (42.9) | .19 |
| Operator | ||||
| L | 52 (51.5) | 47 (54) | 5 (35.7) | .20 |
| A | 30 (29.7) | 26 (29.9) | 4 (28.6) | 1 |
| B | 4 (4) | 3 (3.4) | 1 (7.1) | .46 |
| V | 13 (12.9) | 10 (11.5) | 3 (21.4) | .38 |
| S | 2 (1.9) | 1 (1.1) | 1 (7.1) | .26 |
| Contrast medium type: | ||||
| Iomeprol 350 | 9 (8.9) | 7 (8) | 2 (14.3) | .61 |
| Iohexol 350 | 13 (12.9) | 11 (12.6) | 2 (14.3) | 1 |
| Iodixanol 320 | 79 (78.2) | 69 (79.3) | 10 (71.4) | .50 |
| Contrast medium dose delivered, mL | 242 [189-300] | 240 [188-306] | 258 [195-277] | .95 |
| Total contrast medium dose [delivered plus saved], mL | 355 ± 110 | 354 ± 79 | 356 ± 106 | .95 |
| IVUS use | 24 (23.8) | 23 (26.4) | 1 (7.1) | .18 |
Data are expressed as No. (%), mean ± standard deviation, or median [first quartile-third quartile]. CABG, coronary artery bypass graft; CI-AKI, contrast induced-acute kidney injury; CTO, chronic total occlusion; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasound. | ||||
Table 3. Laboratory, instrumental, clinical characteristics, and Mehran score of cases and controls in the matched group
| Characteristics | No DyeVert (n = 49) | DyeVert (n = 49) | P | Standardized mean difference |
|---|---|---|---|---|
| Laboratory and istrumental characteristics | ||||
| eGFR, mL/min | 53 ± 18 | 51 ± 18 | .70 | 0.11 |
| HCT | 37.8 ± 4.1 | 38.2 ± 4.9 | .68 | 0.08 |
| EF | 50 [40-55] | 50 [35-55] | .68 | 0.13 |
| CKD [eGFR < 60 mL/min/1.73 m2] | 36 (73.5) | 34 (69.4) | .82 | 0.09 |
| Anemia [male HCT < 39, Female HCT < 36] | 27 (55.1) | 24 (49) | .69 | 0.12 |
| Clinical characteristics | ||||
| Age, years | 75 ± 9 | 75 ± 9 | .96 | 0.01 |
| Age > 75 years | 26 (53.1) | 24 (49) | .84 | 0.08 |
| Male sex | 38 (77.6) | 41 (83.7) | .61 | 0.15 |
| Overweight [body mass index ≥ 25] | 22 (44.9) | 24 (49) | .84 | 0.08 |
| Hypertension | 33 (67.3) | 37 (75.5) | .50 | 0.19 |
| Diabetes | 19 (38.8) | 20 (40.8) | 1 | 0.04 |
| Dyslipidemia | 28 (57.1) | 32 (65.3) | .53 | 0.17 |
| Current smoker | 11 (22.4) | 10 (20.4) | 1 | 0.05 |
| Former smoker | 16 (32.7) | 18 (36.7) | .83 | 0.09 |
| CHF [NYHA class ≥ 3 and/or history of pulmonary edema] | 15 (30.6) | 15 (30.6) | 1 | < 0.01 |
| Acute coronary syndrome presentation | 27 (55.1) | 25 (51) | .84 | 0.08 |
| Hypotension [systolic pressure < 80 mmHg for ≥ 1 h requiring inotrope] | 2 (4.1) | 2 (4.1) | 1 | < 0.01 |
| Mehran score | ||||
| Mehran CI-AKI risk class: | ||||
| Low | 12 (24.5) | 10 (20.4) | .63 | 0.09 |
| Moderate | 12 (24.5) | 15 (30.6) | .50 | 0.14 |
| High | 13 (26.5) | 15 (30.6) | .65 | 0.09 |
| Very high | 12 (24.5) | 9 (18.4) | .46 | 0.16 |
| Mehran score, points | 11 ± 6 | 11 ± 6 | .86 | 0.04 |
Data are expressed as No. (%), mean ± standard deviation, or median [first quartile-third quartile]. CHF, congestive heart failure; CI-AKI, contrast induced-acute kidney injury; CKD, chronic kidney disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HCT, hematocrit; NYHA, New York Heart Association. | ||||
Table 4. Procedural characteristics of cases and controls in the matched group
| Characteristics | No DyeVert (n = 49) | DyeVert (n = 49) | P | Standardized mean difference |
|---|---|---|---|---|
| Procedural characteristics (angiography/PCI complexity/complications) | ||||
| Previous CABG | 8 (16.3) | 6 (12.2) | .56 | 0.10 |
| CTO [complete blockage of a coronary artery lasting at least 3 months] | 6 (12.2) | 8 (16.3) | .77 | 0.13 |
| No. vessels treated in the same procedure: | ||||
| 1 | 32 (65.3) | 29 (59.2) | .53 | 0.12 |
| 2 | 15 (30.6) | 17 (34.7) | .67 | 0.08 |
| 3 | 2 (4.1) | 3 (6.1) | 1 | 0.10 |
| No. bifurcations treated in the same procedure: | ||||
| 0 | 33 (67.3) | 31 (63.3) | .67 | 0.09 |
| 1 | 15 (30.6) | 16 (32.7) | .83 | 0.04 |
| 2 | 1 (2.1) | 2 (4) | 1 | 0.12 |
| Left main treatment | 12 (24.5) | 13 (26.5) | 1 | 0.05 |
| Stent, number | 2 [1-3] | 2 [1-3] | .30 | 0.15 |
| Stent lenght, mm | 46 [30-85] | 52 [33-97] | .41 | 0.13 |
| Perforation | 2 (4.1) | 1 (2) | 1 | 0.12 |
| IABP use | 0 (0) | 1 (2) | 1 | 0.20 |
| Rotablator use | 0 (0) | 2 (4.1) | .49 | 0.24 |
| Procedural characteristics (others) | ||||
| Radial access | 41 (83.7) | 45 (91.8) | .35 | 0.24 |
| Femoral access | 11 (22.4) | 15 (30.6) | .49 | 0.18 |
| Operator: | ||||
| L | 24 (49) | 24 (49) | 1 | < 0.01 |
| A | 17 (34.7) | 17 (34.7) | 1 | < 0.01 |
| B | 5 (10.2) | 3 (6.1) | .71 | 0.20 |
| V | 3 (6.1) | 5 (10.2) | .71 | 0.12 |
| Contrast medium type: | ||||
| Iomeprol 350 | 7 (14.3) | 4 (8.2) | .34 | 0.21 |
| Iohexol 350 | 9 (18.4) | 10 (20.4) | .80 | 0.06 |
| Iodixanol 320 | 33 (67.3) | 35 (71.4) | .66 | 0.10 |
| IVUS use | 10 (20.4) | 11 (22.4) | 1 | 0.05 |
Data are expressed as No. (%), mean ± standard deviation, or median [first quartile-third quartile]. CABG, coronary artery bypass graft; CI-AKI, contrast induced-acute kidney injury; CTO, chronic total occlusion; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasound. | ||||
Image quality was evaluated by operators during the procedures. When quality was inadequate, exclusion of the device from the CM line was allowed for the shortest possible time.
AKI was defined as a rise in the concentration of serum creatinine ≥ 0.3 mg/dL within 48 hours after CM administration from the baseline value obtained before CM injection; further measurements after 48 hours were collected in patients with worsening kidney function; for its prevention, all patients received hydration with sodium chloride 0.9% intravenous solution at a rate of 1 or 0.5 mL/kg/h, as appropriate. The severity of AKI was defined according to Kidney Disease Improving Global Outcome (KDIGO) stages.
The research reported was performed in accordance with recommendations for clinical investigation (Declaration of Helsinki of the World Medical Association, October 2013) and was approved by an ethics committee. We declare that relevant informed consent was obtained from all participants and is available.
Objectives
In the case group, we evaluated the following: a) the amount of CMV saved using DyeVert and image quality; b) the rate and severity of CI-AKI and the rate of in-hospital all-cause death; c) laboratory, instrumental, clinical, and procedural differences in the 2 subgroups defined on the basis of the incidence of AKI; and d) independent predictors of CI-AKI.
In the overall population of the case and control groups, we performed propensity score matching (PSM) to obtain a group of patients with a sufficiently good balance (matched group), in which we evaluated the following: a) differences in CMV, and b) rate and severity of CI-AKI.
Statistical analysis
Categorical variables are expressed as the number and percentage of patients. Continuous parametric data are reported as the mean ± standard deviation and continuous nonparametric data as the median [lower and upper quartile]; for assessment of normality, the Kolmogorov test was used. Patients’ categorical variables were compared using the chi-squared test (with Yates’ correction for continuity in the case of variables with only 2 categories) or the Fisher exact test, as appropriate. The unpaired t-test was used for continuous parametric variables and the Mann-Whitney U-test for continuous nonparametric variables; the same tests were used in the matched group. On univariate analysis, significance was defined as P < .05. To establish the independent predictors of AKI, we performed multivariable logistic regression analysis. Variables were selected according to significance in the univariate analysis. The chosen method was stepwise backward regression with a maximum of 20 iterations. Multicollinearity was assessed with tolerance and variance inflation factor (VIF) values. Receiver operating characteristic (ROC) curves were used to establish the optimal cutoffs of independent predictors for the diagnosis of AKI. To perform PSM, the algorithm used was nearest neighbor matching 1:1 with a caliper size of ± 0.2. Statistical analyses were performed using SPSS for Windows, release 29, with R 4.2 implementation to perform PSM.
RESULTS
Analysis in the case group
A total of 101 patients (median age 74 [68-80] years, male sex 79.2%, CKD 72.3%) underwent PCI with the use of the DyeVert Power XT system.
In the overall population of the case group, mean hematocrit (HCT) was 38.6 ± 4.9 %, median EF was 50% [35%-55%], and mean Mehran score was 11 ± 5 points.
Congestive heart failure (CHF) was present in 37 patients (36.6%), Mehran CI-AKI very high-risk class was present in 17 patients (16.8%) and Mehran CI-AKI low-risk class was present in 24 patients (23.8%) (table 1).
We enrolled 20 patients (19.8%) with previous CABG, 12 (11.9%) with CTO, 34 (33.7%) with bifurcations, 25 (24.8%) with left main coronary artery disease, and 44 (43.6%) with multivessel disease. Delivered CM was 242 (189-300) mL, total CM was 355 ± 110 mL, and saved CM was 114 ± 42 mL, with an average of 32% of the total CMV (table 2). In almost all patients (n = 96, 95% of patients), image quality was adequate, while the device was excluded to make it adequate for the shortest possible time in 5 patients. Without these exclusions, saved CMV would have been slightly higher and with trivial changes with regard to the comparison with controls: 33% of the total, a value derived from patients without exclusions (n = 96).
A total of 14 (13.9%) patients developed CI-AKI (AKI-KDIGO 1, 2, 3: 6.9%, 3%, and 4%, respectively). The results of the univariate analysis for the overall population and according to the incidence of CI-AKI in the case group are reported in table 1, table 2, and figure 1.
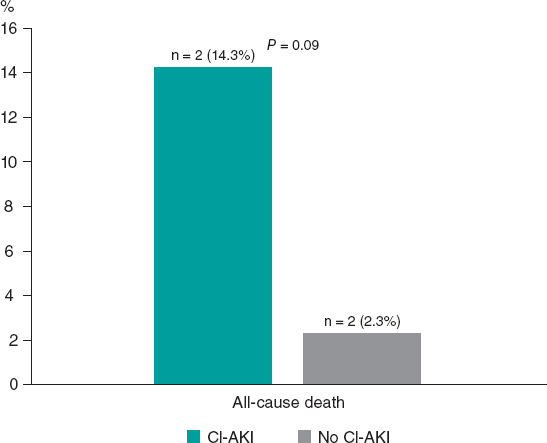
Figure 1. In-hospital all-cause mortality rate according to onset of CI-AKI in the case group. CI-AKI, contrast induced-acute kidney injury.
Compared with patients not developing CI-AKI, those in the CI-AKI subgroup had lower HCT values (35.5 ± 4.8 vs 39.1 ± 4.8; P = .01), lower EF values (30 [28-36] vs 50 [40-55]; P < .001) and higher Mehran score values (15 ± 4 vs 10 ± 5; P < .001).
In addition, the first patients more frequently had CHF [12 (85.7%) vs 25 (28.7%); P <.001] and Mehran CI-AKI very high-risk class (7 [50%] vs 10 [11.5%]; P =.002) and less frequently had Mehran CI-AKI low-risk class (0 [0%] vs 24 [27.6%]; P = .04).
No significant differences were found in the remaining laboratory, instrumental, or clinical features or the procedural variables between the 2 subgroups; in particular, CM was slightly higher in CI-AKI patients: 258 [195-277] vs 240 [188-306] mL, total 356 ± 106 vs 354 ± 79 mL; P = .95 for both variables delivered. In the multivariate analyses, independent predictors of CI-AKI were HCT (OR, 0.86, 95%CI, 0.74-0.99; P = .04) and EF (OR, 0.88, 95%CI, 0.82-0.95; P = .001); the percentage accuracy in classification of the model was 88%, while tolerance and VIF values (0.99 and 1.01, respectively) showed no multicollinearity. The HCT ROC curve showed the following values: area under curve (AUC) 0.71 with 95%CI 0.56-0.87; P = .01; a cutoff of 36.3% had the best sensitivity (72%) and specificity (71%) for the outcome (figure 2). The EF ROC curve showed the following values: AUC 0.83 with 95%CI 0.72-0.94; P = .001; a cutoff of 37% had the best sensitivity (82%) and specificity (79%) (figure 2); therefore, our best predictor was EF < 40%.
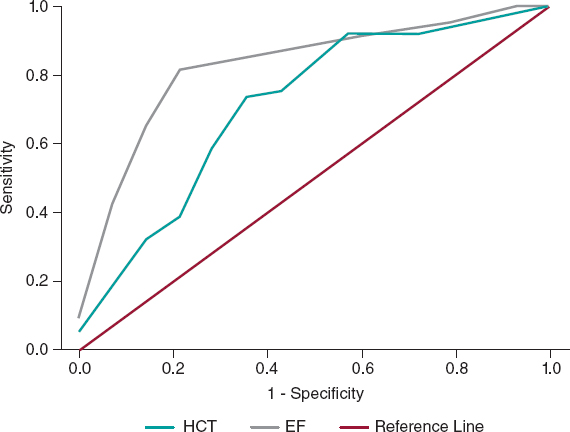
Figure 2. Receiver operating characteristic curves showing the diagnostic ability of HCT and EF for the diagnosis of CI-AKI in the case group. CI-AKI, contrast induced-acute kidney injury; EF, ejection fraction; HCT, hematocrit.
There were 4 in-hospital all-cause deaths overall, 2 deaths in each subgroup (CI-AKI and no–CI-AKI subgroups), as shown in figure 1.
Analysis in the matched group
After the matching process, 49 patients remained in the control (no DyeVert) and case (DyeVert) groups with no significant imbalance (ie, standardized mean differences < ± 0.25), as reported in table 3 and table 4. As shown in figure 3, delivered CM was slightly lower in the DyeVert group than in the no-DyeVert group, with no significant difference (252 ± 80 vs 267 ± 101 mL; P = .42), while total CM was significantly higher in the DyeVert group (354 ± 110 vs 267 ± 101 mL; P < .001). The CI-AKI rate was slightly lower in the case group than in the control group (14.3% vs 16.3%; P = .99) with slightly more advanced stages of AKI in controls (table 1 of the supplementary data), without significance.
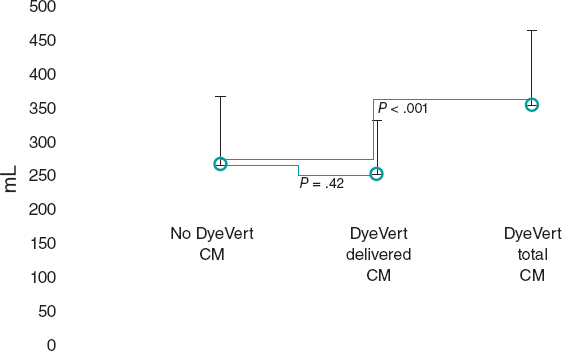
Figure 3. Contrast medium in cases and controls in the matched group. CM, contrast medium; blue dots represent the median values; vertical black lines represent the standard deviations.
DISCUSSION
In the case group, the DyeVert Power XT system saved 32% of CM and image quality was adequate in almost all cases; the only independent predictors of CI-AKI were HCT and EF.
In the matched group, total CM was higher in cases than in controls. After diversion by the device, delivered CM was slightly lower in cases than in controls, but without significance. The reduction in CI-AKI was also nonsignificant.
The DyeVert system is a second-generation device to reduce the amount of CM delivered to patients during angiographic procedures. The first generation was the AVERT system (Osprey Medical Inc), which showed a relative reduction of approximately 23% in CMV among PCI patients compared with controls; the use of the device did not reduce the AKI rate.9 DyeVert Power XT is used in combination with automatic injection; few data are available in this context, being limited to 2 studies that investigated 2610 and 9 patients,11 without a control group. There are more data on manual injection (1696 patients, 15 studies); all these 17 studies were collectively analyzed in the meta-analysis by Tarantini et al.12
In that meta-analysis, the mean saved CMV in the DyeVert group was reported by 7 observational studies and ranged from 34% to 47% of total CMV; the pooled estimate value was approximately 39.5% using manual CM injection systems; of note, the lowest value (34%) was achieved using DyeVert Power XT. We found a similar value in the DyeVert (case) group. These reduced values compared with manual systems may be related to different pressures generated during automatic contrast injection.
In our case group analysis, CMV was not significantly correlated with the occurrence of CI-AKI, which instead was independently predicted by lower HCT and EF values, which are known risk factors, as shown by Mehran scores.2,4 EF was also an independent predictor in the study by Briguori et al.13 Our findings confirm the importance of first identifying the variables (eg, those in the Mehran or Mehran 2 scores)2,4 that classify patients at higher risk of CI-AKI to apply appropriate preventive strategies. In the present study, these patients were identified by HCT and EF and consequently the latter variables (especially EF) may be more important predictors than CMV, which is normally used during PCI in the general population. In the above-mentioned scores, CMV was also an independent predictor of CI-AKI and, consequently, using the smallest possible value of CMV is still important, especially in higher risk patients. DyeVert thus has the potential benefit of reducing CIN, depending on its efficacy compared with controls, which was evaluated in the above-mentioned meta-analysis and in the present study.
In the meta-analysis, approximately half of the studies included controls for comparison. Delivered CM was usually lower in DyeVert patients than in controls. In these cases, the difference ranged from 22 to 50 mL,12 with the highest differences being reported in the studies by Tajti et al. (200 [153-256] vs 250 [170-303] mL; P = .04) and Briguori et al. (99 ± 50 vs 130 ± 50 mL; P <.001).13,14 Delivered CMV was slightly higher (difference of 2 mL) in the DyeVert group only in the study by Bunney et al.15 The pooled analysis showed a significant decrease in delivered CMV with DyeVert use relative to the control group. Of note, details about prior CABG, CTO and left main treatment were reported only in 1 work14 and the number of vessels treated was reported only in another work.13 The treatment of bifurcations and differences in operators were not reported. All these procedural characteristics, which may influence the amount of CM delivered during PCI, were included in our study and we used a matched group with a sufficiently good balance in the studied characteristics.
In our matched group, delivered CM was lower in the case group than in the control group, but the difference was slight and nonsignificant, while total CM (also called attempted in the meta-analysis) was significantly higher in the case group than in the control group. Consequently, the net practical benefit of the device in terms of spared CM was low. In our work, procedural characteristics (eg, procedural complexity), which could cause discrepancies in CM injections, were balanced in the matched group. Based on these findings, we believe that the control group required more prolonged and/or a greater number of contrast injections (and consequently more total CM) to achieve adequate image quality. In previous studies, adequate image quality was achieved with DyeVert in 98% of cases,12 a value similar to ours, but those studies did not discuss the need for prolonged injections and more total CM compared with controls to maintain image quality when DyeVert is used. Few data are available on total CM, but previous studies reporting this information indicate that total CM was higher in DyeVert patients than in controls (Briguori et al., P-value almost significant; Kutschman et al., P-value not reported).12,13,16
The reduction in CI-AKI in the present study was not statistically significant. In the meta-analysis, the pooled relative risk for CI-AKI associated with DyeVert system use was 0.60 (95%CI, 0.40-0.90; P = .01), which was a result derived from 5 studies. Moreover, in a recent abstract not included in the meta-analysis, postprocedure eGFR values among patients undergoing coronary and/or peripheral angiography were significantly more stable in the DyeVert group than in controls.17
Analysis of the 5 above-mentioned studies separately revealed that our results are mainly in agreement; indeed, the relative risk was significantly lower in only 1 study in the nonpooled analysis.13
The type of CM was not associated with the occurrence of CI-AKI; as recommended,18 we used iso-osmolar (Iodixanol 320) or low-osmolar (Iomeprol 350 or Iohexol 350) contrast agents to prevent CIN. Given the presence of more favorable evidence,19 we preferred to use the iso-osmolar agent and reserved the other agents to low-risk patients.
Study limitations
Our study has some limitations. First, the sample size was relatively small. Second, the study design was single center, observational and retrospective, although we performed PSM to reduce potential confounding bias. Third, we excluded patients not meeting the inclusion criteria, as they were usually at low risk of CI-AKI. Therefore, our results should be generalized with caution, since the analyzed patients may be not representative of the general population. In this work the variable of sex has not been taken into account in accordance with the SAGER guidelines.
CONCLUSIONS
The DyeVert Power XT system saved 32% of CM, but only HCT and EF were independent predictors of CI-AKI and the main predictor was EF < 40%. Therefore, these variables (especially EF) may be more important than CMV, which is normally used during PCI in the general population.
PCI with this system required more total CM compared with that in controls to achieve adequate image quality. Consequently, after CM saving by the device, delivered CM was only slightly lower than CM in controls (mean difference of 15 mL) and this difference was nonsignificant. Therefore, the net practical benefit of the system was low. Equally, the reduction in CI-AKI (14.3% vs 16.3%) was not statistically significant.
Future studies are needed to confirm these results.
FUNDING
The authors did not receive any grants for this research.
ETHICAL CONSIDERATIONS
The work has been approved by an Ethics Committee/institution. Informed consent of patients was obtained and archived for the publication of their cases. In this work the variable of sex has not been taken into account in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
We didn’t use artificial intelligence for the development of our work.
AUTHORS’ CONTRIBUTIONS
F. Vergni, M.Arioti, and M.Leoncini contributed to the design of the work. F. Vergni, M. Arioti, V. Boasi, F.A. Sánchez, M.Leoncini, and F. Ferrari contributed to the acquisition of data. F. Vergni analyzed the data. F. Vergni, M.Arioti, V. Boasi, F.A. Sánchez, M. Leoncini, and F. Ferrari contributed to the interpretation of the data. F. Vergni and M.Arioti contributed to the drafting of the work. F. Vergni, M. Arioti, V. Boasi, F.A. Sánchez, M.Leoncini, and F. Ferrari revised the work and approved the final version to be published.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
What is known about the topic?
- The DyeVert Power XT system (which is used in conjunction with automated contrast injection) has been assessed in only 2 studies, which included a total of 35 patients investigated without a control group and mainly not during PCI.
What does this study add?
- Our study investigated the device in a larger population (n = 101) and during PCI. Moreover, we included a control group and performed propensity score matching to obtain a group of patients with a sufficiently good balance regarding laboratory, instrumental, clinical and procedural characteristics; in addition, among the latter features, we included the treatment of coronary bifurcations and differences between operators, which were not reported in previous studies. The above-mentioned characteristics may influence the outcome (ie, CI-AKI occurrence) and/or the volume of CM used and therefore their inclusion is important when assessing a device to spare CM.
REFERENCES
1. Lun Z, Liu L, Chen G, et al. The global incidence and mortality of contrast-associated acute kidney injury following coronary angiography:a meta-analysis of 1.2 million patients. J Nephrol. 2021;34:1479-1489.
2. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention:development and initial validation. J Am Coll Cardiol. 2004;44:1393-1399.
3. Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy:from pathophysiology to preventive strategies. Can J Cardiol. 2016;32:247-255.
4. Mehran R, Owen R, Chiarito M, et al. A contemporary simple risk score for prediction of contrast-associated acute kidney injury after percutaneous coronary intervention:derivation and validation from an observational registry. Lancet. 2021;398:1974-1983.
5. Almendarez M, Gurm HS, Mariani J Jr, et al. Procedural strategies to reduce the incidence of contrast-induced acute kidney injury during percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1877-1888.
6. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
7. Desch S, Fuernau G, Pöss J, et al. Impact of a novel contrast reduction system on contrast savings in coronary angiography –the DyeVert randomised controlled trial. Int J Cardiol. 2018;257:50-53.
8. Zimin VN, Jones MR, Richmond IT, et al. A feasibility study of the DyeVerttm plus contrast reduction system to reduce contrast media volumes in percutaneous coronary procedures using optical coherence tomography. Cardiovasc Revasc Med. 2021;30:40-46.
9. Mehran R, Faggioni M, Chandrasekhar J, et al. Effect of a contrast modulation system on contrast media use and the rate of acute kidney injury after coronary angiography. JACC Cardiovasc Interv. 2018;11:1601-1610.
10. Amoroso G, Christian J, Christopher A. First European experience using a novel contrast reduction system during coronary angiography with automated contrast injection. [Abstract]. Eurointervention. 2020;16(Suppl. AC):Euro20A-POS426.
11. Bruno RR, Nia AM, Wolff G, et al. Early clinical experiences with a novel contrast volume reduction system during invasive coronary angiography. Int J Cardiol Heart Vasc. 2019;23:100377.
12. Tarantini G, Prasad A, Rathore S, et al. DyeVert Contrast Reduction System Use in Patients Undergoing Coronary and/or Peripheral Angiography:A Systematic Literature Review and Meta-Analysis. Front Med (Lausanne). 2022;9:841876.
13. Briguori C, Golino M, Porchetta N, et al. Impact of a contrast media volume control device on acute kidney injury rate in patients with acute coronary syndrome. Catheter Cardiovasc Interv. 2021;98:76-84.
14. Tajti P, Xenogiannis I, Hall A, et al. Use of the DyeVert system in chronic total occlusion percutaneous coronary intervention. J Invasive Cardiol. 2019;31:253-299.
15. Bunney R, Saenger E, Shah C, et al. Contemporary use of contrast dye reduction technology in a tertiary academic hospital:patient characteristics and acute kidney injury outcomes following percutaneous coronary interventions. In:Acc 2019. 1st Quality Summit;2019 March 13-15;New Orleans, United States.
16. Kutschman R, Davison L, Beyer J. Comprehensive clinical quality initiative for reducing acute kidney injury in at-risk patients undergoing diagnostic coronary angiogram and/or percutaneous coronary interventions. In:Scai 2019. 42nd Scientific Sessions;2019 May 19-22;Las Vegas, United States.
17. Olubowale O, Ur Rahman E, U Okoro K, et al. The DyeVert contrast reduction system and contrast induced nephropathy:is it any better?J Am Coll Cardiol. 2022;79(Suppl 9):S903.
18. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
19. Zhao F, Lei R, Yang SK, et al. Comparative effect of iso-osmolar versus low-osmolar contrast media on the incidence of contrast-induced acute kidney injury in diabetic patients:a systematic review and meta-analysis. Cancer Imaging. 2019;19:38.
* Corresponding author.
E-mail address: Vergni95@gmail.com (F. Vergni).











