Thirty-six-year-old male referred to the emergency room by the general practitioner with chest pain of 1-month duration. After the initial assessment revealed the presence of diffuse T-wave flattening, normal troponin levels, and a normal echocardiogram, the patient was discharged with a diagnosis of low-risk atypical chest pain. However, due to symptom recurrence and the fact that the treadmill test triggered angina-like symptoms and the ECG showed transient T-wave abnormalities, a coronary angiography was performed that ruled out the presence of epicardial stenosis (video 1 of the supplementary data).
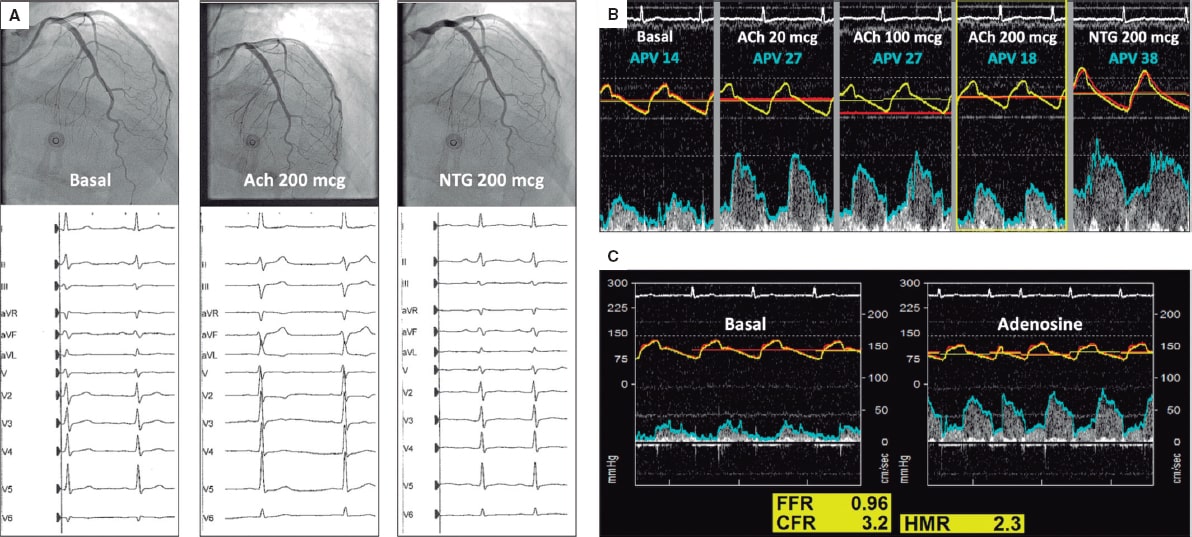
The patient was readmitted a week later due to disabling oppressive chest pain related to low intensity exercise. Due to persistent angina without coronary stenosis, an intracoronary physiological assessment was scheduled with acetylcholine, adenosine, and simultaneous pressure and flow measurements in the left anterior descending coronary artery using a dual-sensor pressure and Doppler velocity guidewire. Acetylcholine (Ach) (200 mcg) triggered the patient’s typical symptoms, the repolarization abnormalities seen on the ECG and the 50% decrease of coronary artery flow velocity (APV) without epicardial spasm (video 2 of the supplementary data). Intracoronary nitroglycerin (NTG) solved all abnormalities (figure 1A,B). The adenosine non-endothelium-dependent assessment revealed a normal fractional flow reserve (FFR), hyperemic microcirculatory resistance (HMR), and coronary flow reserve (CFR) (figure 1C). Because of the clinical features present (acetylcholine-triggered angina with repolarization abnormalities seen on the ECG without epicardial spasm), microvascular vasospastic angina was diagnosed. Medical therapy adjustment with calcium channel blockers as first-line treatment improved the patient’s symptoms at the follow-up.
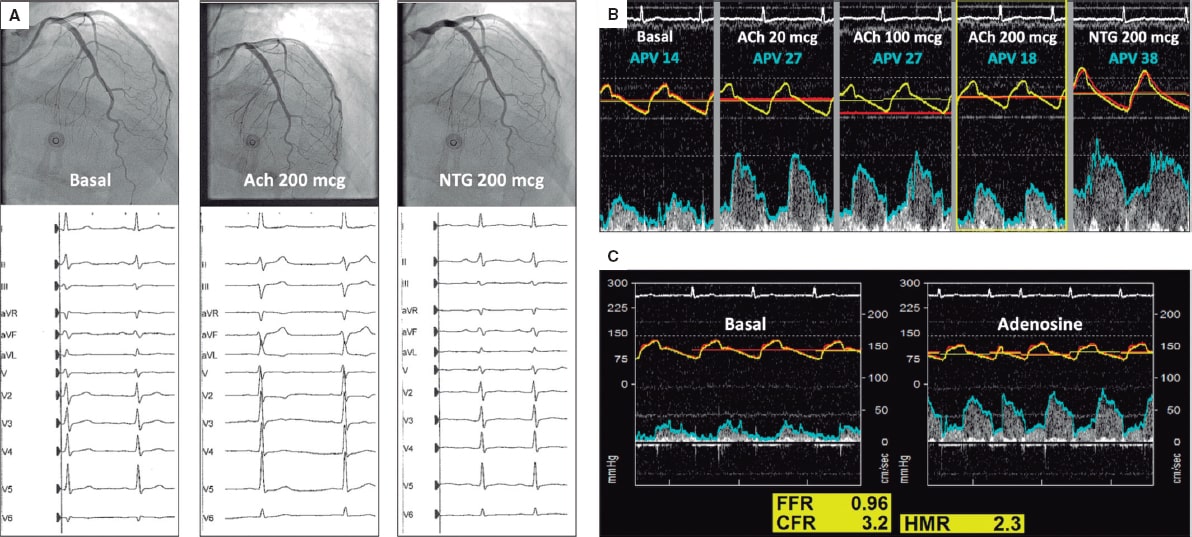
Figure 1.
This case illustrates the value of comprehensive intracoronary physiological assessment to evaluate specific pathways of vascular dysfunction in patients with persistent angina. This approach is supported by the 2019 ESC clinical practice guidelines on the management of chronic coronary syndromes in patients without obstructive coronary stenoses. The patient’s consent was obtained to report his case anonymously.
FUNDING
The manuscript has not been funded.
AUTHORS' CONTRIBUTIONS
The text has been prepared and revised with the participation of all signatory authors.
CONFLICTS OF INTEREST
None declared.
SUPPLEMENTARY DATA
Vídeo 1. Provencio A. DOI: 10.24875/RECICE.M20000180
Vídeo 2. Provencio A. DOI: 10.24875/RECICE.M20000180
Corresponding author: Servicio de Cardiología, Hospital Clínico San Carlos, Prof. Martín Lagos S/N, 28040 Madrid, Spain.
E-mail address: hmejiarenteria@gmail.com (H. Mejía-Rentería).
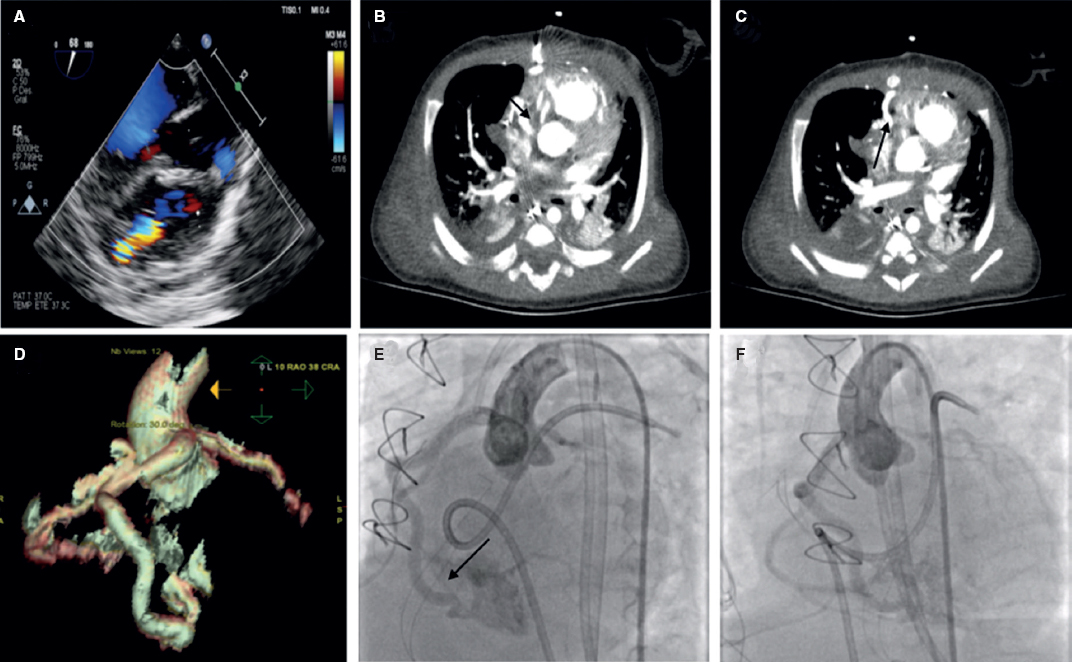
Seven-year-old male with single ventricle in the postoperative of a fenestrated extracardiac Fontan procedure. The echocardiography performed 7 days after the procedure confirmed the presence of a 20 mm pedunculated thrombus attached to the wall of the inferior vena cava (video 1 of the supplementary data).
Due to the risk of systemic embolism through the Fontan fenestration, the percutaneous extraction of the thrombus was attempted using an Amplatzer Vascular Plug II (AVP II) device (Abbott, United States).
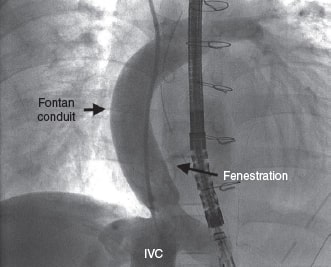
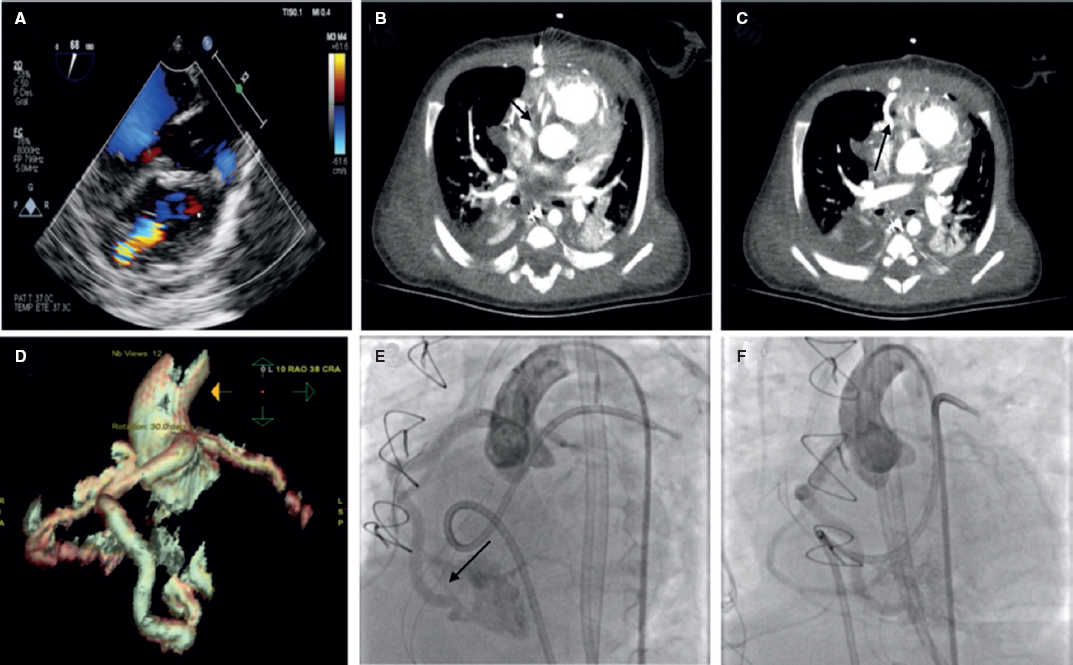
A 6-Fr introducer sheath and a 10-Fr sheath were used for the catheterization of the right jugular vein and right femoral vein, respectively. The fenestration was found in the Fontan conduit using an angiography (figure 1). It was catheterized via jugular access and occluded by inflating the balloon of a 6-Fr Wedge-Pressure Catheter (Arrow, United States) to protect an eventual paradoxical embolism (figure 2). The transesophageal echocardiography performed revealed the location of the thrombus in the inferior vena cava and the diameter of the cava was measured at that level (15 mm). The introducer sheath was advanced via femoral access until it landed right underneath the insertion of the thrombus. Through this sheath a folded 16 mm AVP II device was advanced inside an 8-Fr multipurpose catheter that was advanced until it passed the thrombus distal edge. The AVP II was unfolded in this position and pulled back until it was finally inserted into the sheath located in the inferior vena cava (figure 3 and videos 2, 3, and 4 of the supplementary data). Although the thrombus was never retrieved, it disappeared leaving no trace of pulmonary embolism. The consent of the patient’s father was obtained for the publication of the case.
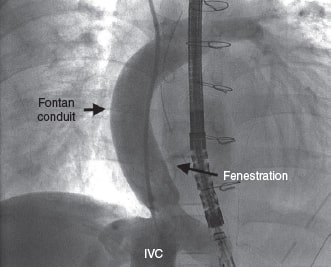
Figure 1.
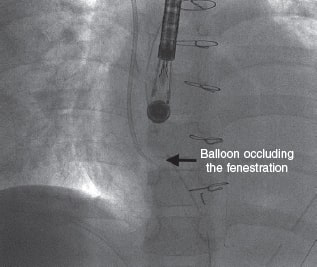
Figure 2.
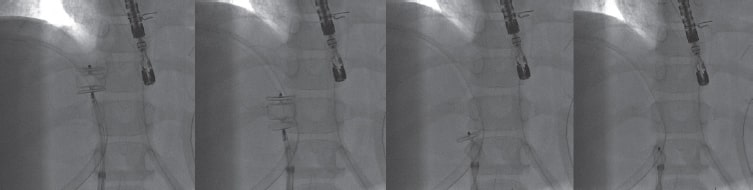
Figure 3.
FUNDING
None.
AUTHORS' CONTRIBUTIONS
All the authors have participated in the writing of this article and have read and approved the final version.
CONFLICTS OF INTEREST
None.
SUPPLEMENTARY DATA
Vídeo 1. Mendoza A. DOI: 10.24875/RECICE.M20000182
Vídeo 2. Mendoza A. DOI: 10.24875/RECICE.M20000182
Vídeo 3. Mendoza A. DOI: 10.24875/RECICE.M20000182
Vídeo 4. Mendoza A. DOI: 10.24875/RECICE.M20000182
Corresponding author: Instituto Pediátrico del Corazón, Hospital Universitario 12 de Octubre, Avda. de Córdoba s/n, 28041 Madrid, Spain.
E-mail address: alberto.mendoza@salud.madrid.org (A. Mendoza).
Sixty-six-year-old male patient with a past medical history of mitral and aortic valve replacement in 1983. Back in 2005 he underwent a new aortic valve replacement due to prosthetic valve dysfunction. In 2018, also due to prosthetic valve dysfunction, a new mitral valve replacement was performed with a size 27 Bicarbon Fitline heart valve (Sorin Group, Italy).
Three months later the patient was hospitalized with functional class III-IV heart failure according to the New York Heart Association (NYHA) and hemolytic anemia with multiple mitral paravalvular leaks quantified as severe regurgitation. In a single medical-surgical session it was decided to perform percutaneous treatment due to the patient’s high surgical risk. The percutaneous closure of the leaks was performed using 7 Amplatzer Vascular Plug III devices (figure 1E) that resulted in minimal residual leaks.
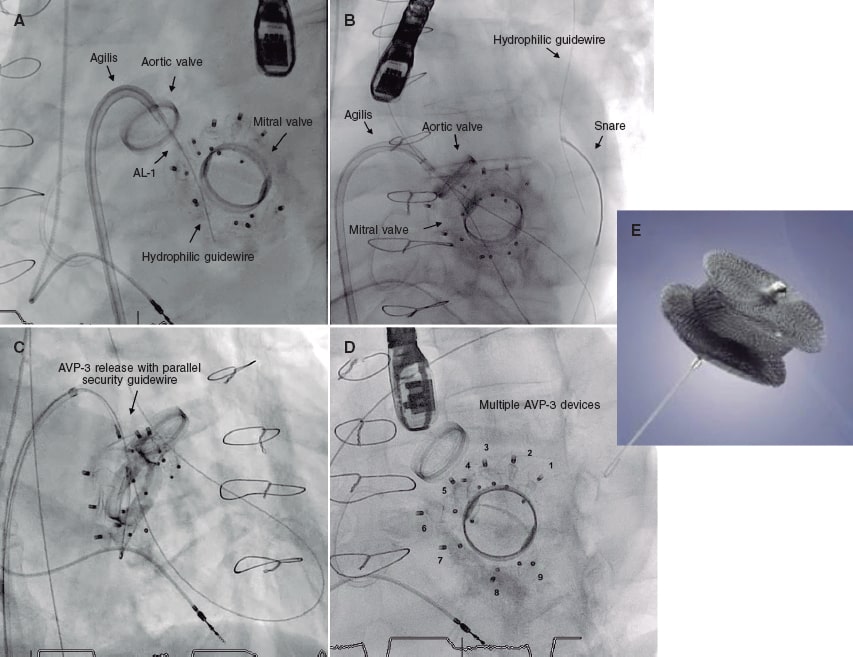
In 2019 the patient presented with NYHA functional class III-IV and hemolytic anemia back again. The 3D echocardiogram performed revealed the presence of 2 severe mitral paravalvular leaks (figure 2A,B) (video 1 of the supplementary data). Percutaneous approach was attempted. An antegrade venoarterial circuit via transseptal puncture was built using a 0.035 in hydrophilic guidewire (figure 1A,B) (videos 2 and 3 of the supplementary data), 2 12/5 mm Amplatzer Vascular Plug III devices were implanted (figure 1C) (videos 4 and 5 of the supplementary data), and 9 devices of the same type surrounding the prosthetic circumference (figure 1D and figure 2D) (video 6 of the supplementary data). The procedure was completed uneventfully. The transesophageal echocardiography performed during the procedure confirmed the reduction of mitral paravalvular leaks now quantified as mild regurgitation (figure 2C) (video 7 of the supplementary data). Disease progression was good, and the patient was discharged with NYHA functional class II-III, corrected anemia (hemoglobin of 12 g/dL), and outpatient follow-up.
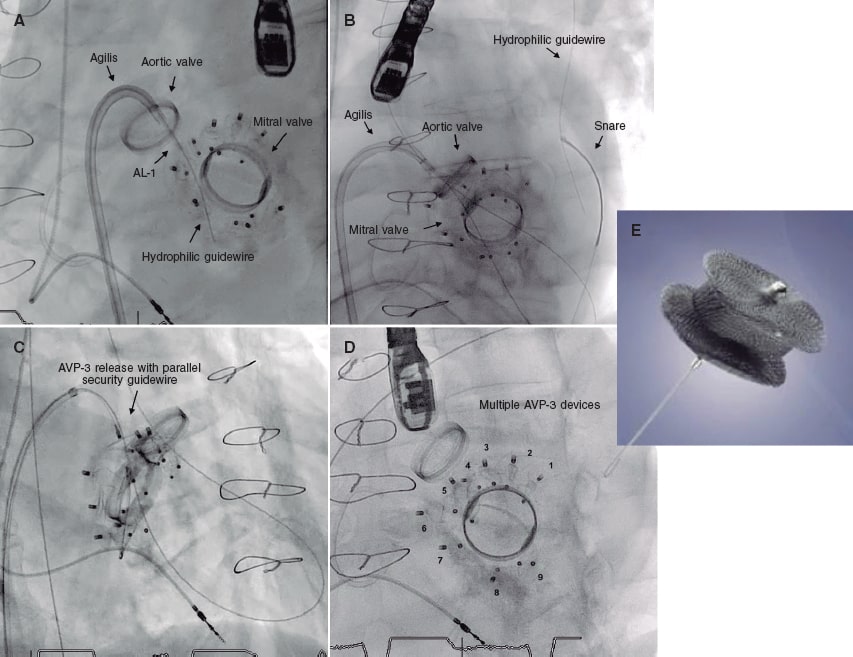
Figure 1.
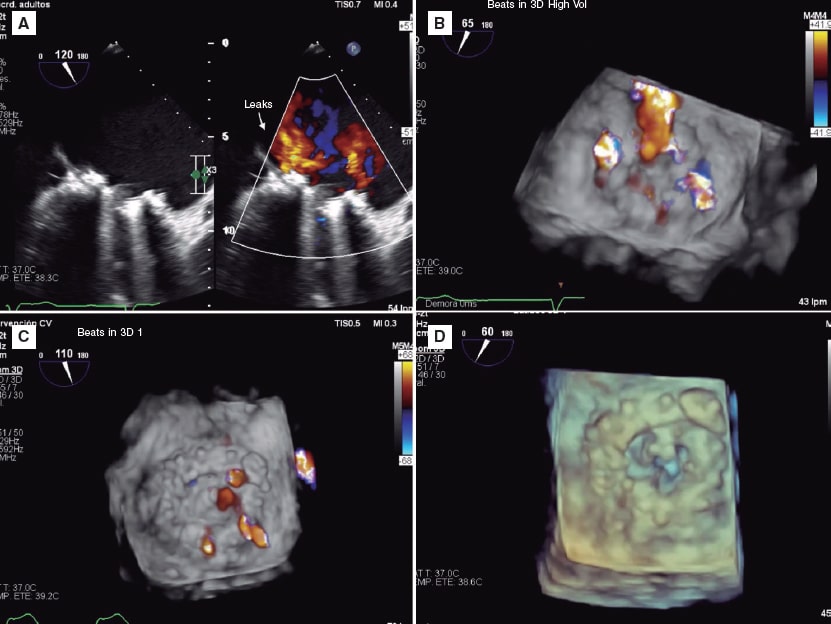
Figure 2.
CONFLICTS OF INTEREST
I. Cruz Gónzalez is proctor for Abbott.
SUPPLEMENTARY DATA
Video 1. Gómez Silva M. DOI: 10.24875/RECICE.M20000166.
Video 2. Gómez Silva M. DOI: 10.24875/RECICE.M20000166.
Video 3. Gómez Silva M. DOI: 10.24875/RECICE.M20000166.
Video 4. Gómez Silva M. DOI: 10.24875/RECICE.M20000166.
Video 5. Gómez Silva M. DOI: 10.24875/RECICE.M20000166.
Video 6. Gómez Silva M. DOI: 10.24875/RECICE.M20000166.
Video 7. Gómez Silva M. DOI: 10.24875/RECICE.M20000166.
* Corresponding author: Unidad de Hemodinámica, Hospital Dr. Gustavo Fricke, Avda. Álvarez 1532, Viña del Mar, Valparaíso, Chile.
E-mail address: marceloalejandro.gomezsilva@gmail.com (M. Gómez Silva).
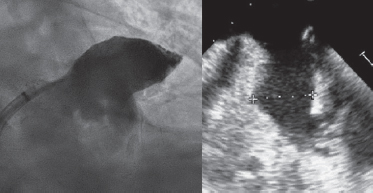
An 80-year-old male with permanent atrial fibrillation (informed consent obtained) underwent a percutaneous procedure to close the left atrial appendage (LAA). He had required repeated admissions for severe anemia and chronic gastrointestinal bleedings while on different antithrombotic regimens (aspirin alone, clopidogrel alone, apixaban). He had a CHADS-VASC2 score of 6 and a HAS-BLED score of 4. A transesophageal echocardiography (TEE) performed revealed the presence of Windsock morphology and no thrombus in the LAA. The diameters of the landing zone were between 23 mm and 25 mm (figure 1).
Figure 1.
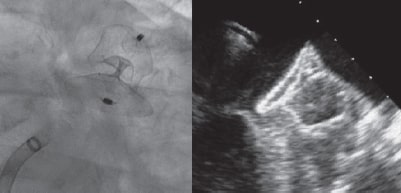
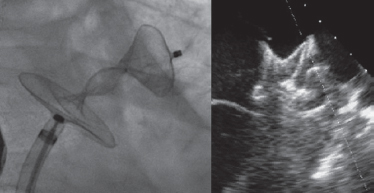
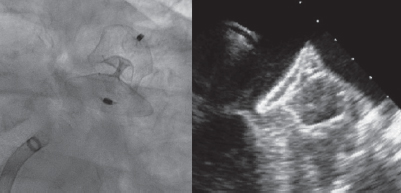
A 28-mm Amulet device was chosen to perform the procedure. A 14-Fr introducer sheath was advanced into the left atrium and, after a selective angiography, the device was deployed inside the LAA in a regular fashion. The first deployment did not achieve a good position (partially outside the appendage) and the device had to be recaptured. A second attempt was made with significant counterclockwise rotation of the sheath that achieved a peculiar “twisted” deployment of the body of the device (figure 2) whose distal part was actually deployed inside the appendage. It was carefully recaptured and after discarding pericardial effusion, it was re-implanted in a good position this time with no further need to change the device (figure 3). A transthoracic echocardiography performed the next day revealed no pericardial effusion. The TEE performed 1 month later revealed no leaks or thrombi on the device either.
Figure 2.
Figure 3.
The immediate complications described with LAA occluding devices are embolization, incomplete closure with residual leaks or the development of pericardial effusion and cardiac tamponade. Twisted malappositions are actually rare and could be predictors of short-term complications.
FUNDING
No funding source related to he manuscript.
AUTHORS' CONTRIBUTION
F. Hernández and E. Lázaro, procedure performance; all authors contributed in the drafting and revision of the manuscript.
CONFLICTS OF INTEREST
F. Hernández Hernández is a proctor for Abbott on issues concerning left atrial appendage occlusions.
Corresponding author: Departamento de Cardiología y Cirugía Cardiaca, Clínica Universidad de Navarra, Marquesado de Santa Marta 1, 28027 Madrid, Spain.
E-mail address: felipeivus@hotmail.com (F. Hernández Hernández).
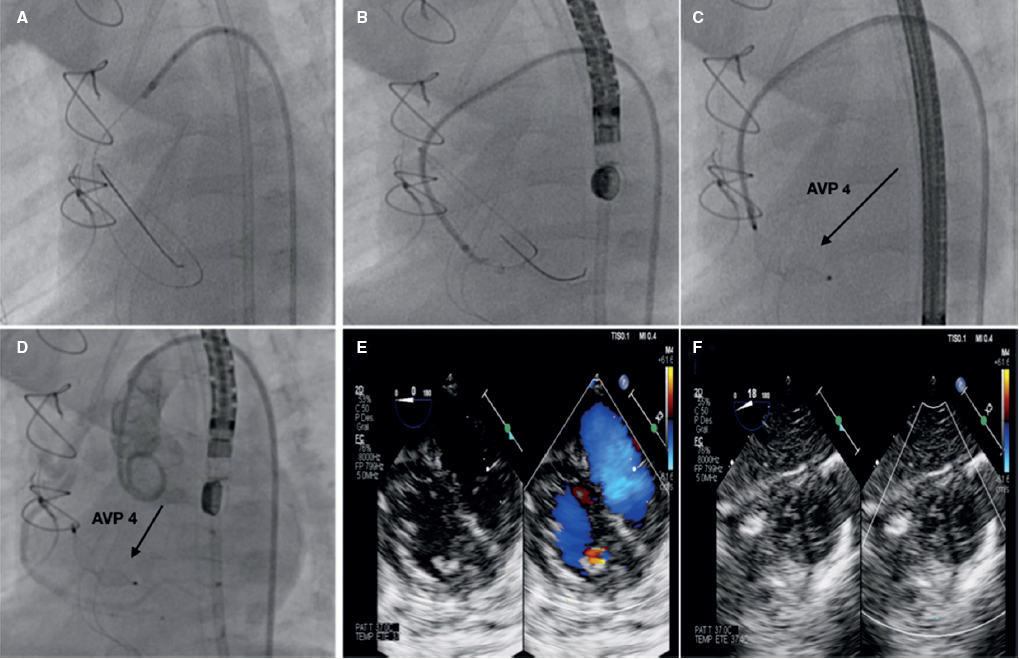
Two-month baby recently treated with closure of perimembranous interventricular communication with congestive clinical signs in the postoperative period and echocardiographic suspicion of possible right coronary artery-to-right ventricle fistula confirmed on the cardiac computed tomography and catheterization with nonselective coronary angiography. Parents' informed consent was obtained for the dissemination of the case. A large coronary fistula—eligible for percutaneous closure—can be seen exiting the right coronary artery marginal branch and entering the right ventricular cavity with great dilatation of the right coronary artery proximal segment (figure 1). A 4-Fr carrier catheter is inserted into the right coronary artery and a guidewire is advanced towards the right ventricle. Assisted by an angioplasty balloon, the carrier catheter is then advanced towards the fistula proximal zone where eventually a 4 × 6 Amplatzer Vascular Plug (AVP4) device (Abbott, United States) is delivered resulting in the total occlusion of the defect with no interference with the distal right coronary artery (figure 2).
Figure 1.
Figure 2.
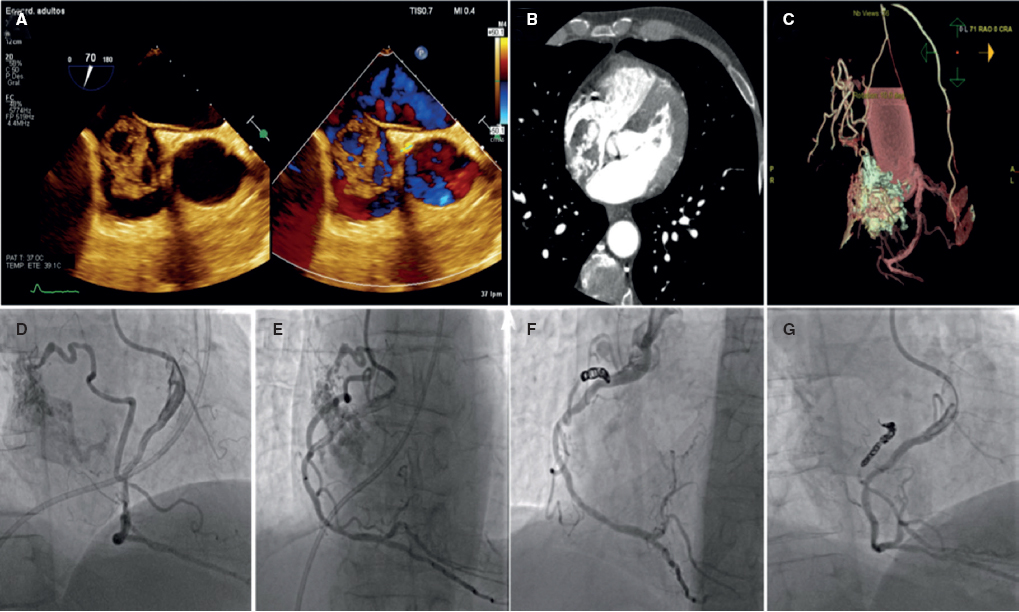
Seventy-year-old male patient with an incidental finding on the transthoracic and transesophageal echocardiography of a large mass in the right atrium with abundant vascular component. Patient' informed consent was obtained for the dissemination of the case. The cardiac CT performed confirmed the finding of an arteriovenous malformation. The coronary angiography performed shows afference from the mid-right coronary artery towards the atrial mass and percutaneous closure is decided. The closure is performed by advancing a microcatheter towards the afferent branch mid-section and protecting the mid-right coronary artery through the inflation of the angioplasty balloon. Ten Hilal Coils (Cook Medical, United States) are delivered with good results and complete closure of the defect (figure 3).
Figure 3.
FUNDING
No funding to declare.
AUTHORS' CONTRIBUTION
All authors have contributed to the elaboration, drafting and revision of the manuscript.
CONFLICTS OF INTEREST
None declared.
Corresponding author: Unidad de Cardiología Intervencionista, Plaza Cruces s/n, 48903 Baracaldo, Vizcaya, Spain.
E-mail address: luisfg82@hotmail.com (L. Fernández González).
Editorials
Transcatheter aortic valve replacement for noncalcified aortic regurgitation. Where are we now?
aServicio de Cardiología, Hospital Clínico Universitario, Valladolid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain
Original articles
Editorials
Vascular closure devices: the jury is still out
aUnidad de Hemodinámica, Servicio de Cardiología, Hospital General Universitario Dr. Balmis, Instituto de Investigación Sanitaria y Biomédica de Alicante (ISABIAL), Alicante, Spain
bDepartamento de Medicina Clínica, Universidad Miguel Hernández, Alicante, Spain
Original articles
Debate
Debate: Asymptomatic severe aortic stenosis: when should we intervene?
The clinician’s perspective
Servicio de Cardiología, Hospital Ramón y Cajal, Madrid, Spain
The interventional cardiologist’s perspective
Unidad de Cardiología Intervencionista, Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo, Vigo, Pontevedra, Spain