ABSTRACT
Introduction and objectives: Although cardiac catheterization (CC) has become a routine practice in pediatric heart transplantation (HT), there is still a shortage of widely used protocols and strong evidence on the number of procedures required and their impact on HT outcomes, as well as the need for further CC. This study aimed to analyze CC activity in pediatric HT recipients in a tertiary center and describe risk factors for a higher number of post-HT procedures.
Methods: This retrospective study obtained data from medical reports and image files. The sample was composed of patients with cardiomyopathies and congenital heart diseases (CHD). Risk factor analysis for CCs was conducted with linear regression and the ANOVA test.
Results: The sample included 61 children (36.07% with CHD). The CHD group had a higher mean number of CCs prior to HT. The most frequent activities prior to HT were diagnostic catheterizations, followed by endomyocardial biopsies for cardiomyopathies and aortopulmonary collaterals in CHD patients. There were 389 post-HT CCs (608 procedures). Most CCs were performed for rejection surveillance, accounting for 92.75% of procedures. The univentricular CHD subgroup was associated with a higher number of CC after HT (P = .03).
Conclusions: Despite long life expectancy, pediatric HT recipients have substantial morbidity due to these procedures. Therefore, it is necessary to establish protocols for follow-up and rejection surveillance to minimize the interventions required by these patients.
Keywords: Pediatric heart transplantation. Cardiac catheterization. Graft rejection. Endomyocardial biopsy.
RESUMEN
Introducción y objetivos: A pesar de que el cateterismo cardiaco (CC) se ha convertido en una práctica habitual en el trasplante cardiaco (TxC) pediátrico, hay escasez de protocolos globales y de evidencia robusta sobre los procedimientos requeridos y el impacto que tienen en la evolución del propio trasplante y los futuros CC. Este estudio tiene como objetivo analizar la actividad de CC en niños receptores de trasplante cardiaco en un centro terciario y describir los factores de riesgo para un mayor número de procedimientos.
Métodos: Estudio retrospectivo con datos obtenidos de los informes médicos y los archivos de hemodinámica. La muestra se dividió en miocardiopatías y cardiopatías congénitas (CAC). El análisis de los factores de riesgo para CC se calculó con regresión lineal y ANOVA.
Resultados: Conformaron la muestra 61 niños (36,07% CAC). Las CAC muestran una mayor media de CC antes del TC. Los cateterismos diagnósticos son la actividad más frecuente previa al TxC, seguidos por las biopsias endomiocárdicas en las miocardiopatías y el cierre de colaterales aortopulmonares en las CAC. Hubo 389 CC tras el TC (608 procedimientos), la mayoría (92,75%) por vigilancia del rechazo. El subgrupo de CAC univentriculares tuvo significativamente un mayor número de CC tras el TxC (p = 0,03).
Conclusiones: A pesar de su larga expectativa de vida, los niños receptores de TxC sufren una morbilidad importante debido a los CC, por lo que es necesario establecer protocolos de seguimiento y vigilancia del rechazo para minimizar las intervenciones que necesitarán.
Palabras clave: Trasplante cardiaco pediátrico. Cateterismo cardiaco. Rechazo injerto. Biopsia endomiocárdica.
Abbreviations CC: cardiac catheterization. CHD: congenital heart disease. EMB: endomyocardial biopsy. HT: heart transplant. IVUS: intravascular ultrasound.
INTRODUCTION
Heart transplant (HT) in children is an uncommon but complex procedure that entails close chronic monitoring. HT involves not only major surgery and challenging postsurgical recovery but also requires lifelong rejection surveillance and review of anastomosis or surgical complications. The latter can be assessed through cardiac catheterization (CC) studies, which have become an indispensable practice in HT follow-up.
Some authors have studied whether the underlying disease can influence the course of post-HT surgery. Although the literature reports discrepant controversial results, it is generally observed that patients with congenital heart diseases (CHD) have higher rates of post-HT procedures regardless of the number of previous CCs. This could be because diagnostic procedures are rare in pediatrics, and therefore, the therapeutic catheterizations reported were mainly performed in patients with congenital disease. It is also well-known that younger recipients (especially if aged < 1 year) and those with hypoplastic left heart syndrome (HLHS) require a larger number of interventions.1-3
Globally, endomyocardial biopsy (EMB) is the most frequently performed diagnostic procedure. EMB is the gold standard test for diagnosing rejection, as noninvasive tests are not currently available. Coronary angiography allows monitoring for coronary allograft vasculopathy (CAV), which is a marker of chronic rejection, the leading cause of death beyond the third year post-HT. Coronary intravascular ultrasound (IVUS) is an advanced complementary tool for the detection and grading of CAV.4,5
Reported interventions after HT mainly include the treatment of aortic arch and systemic vein connection to right atrium stenosis. The latter is mainly associated with lower weight, a greater donor-recipient size discrepancy, and more frequent complex anatomies.6,7
Other indications for CC include haemodynamic assessment for congestive symptoms and diagnosis of pulmonary hypertension, which are of the utmost importance as they are markers of the need for retransplantation.8,9
Due to the rarity of pediatric HT, most centers are developing protocols for the frequency at which these CCs should be performed and how the technique should be applied, with the aim of establishing common practice and achieving better results. Therefore, knowledge of the procedures performed is essential.
The objective of the present study was, in first instance, to determine CC activity after pediatric HT and, second, to study the risk factors for higher post-HT CC requirements, based on the medical history and previous procedures.
METHODS
This retrospective study included all pediatric HT recipients aged < 18 years at the time of HT who underwent at least 1 post-HT CC in a university tertiary hospital from 2002 to 2021. The study was approved by the local ethics committee, with consent form exemption, and was performed in accordance with the principles of the Declaration of Helsinki.
The data reviewed include patients’ medical history on previous CCs and surgical interventions, demographic information, and complications during the HT surgery. For all post-HT CCs, we collected data on the material used, timing, specific procedures, and diagnoses for each participant. Due to differences in their presentation and progression, patients with cardiomyopathies and CHD were analyzed separately in some of the analyses. A procedure was defined as any intervention/technique performed, while each visit to the catheterization laboratory was considered a separate CC.
Given the retrospective nature of the study and the patient age group, the patients’ gender was extracted from the documented sex assigned at birth or from their medical history.
Qualitative data are expressed as percentages, while the mean and standard deviation (SD), or median and interquartile range (IQR) are used for quantitative variables. Differences were analyzed by the Fisher, Chi-square, Mann-Withney U or T-students tests, depending on the type of variable. Risk factors for increased post-HT requirements were examined using linear regression or ANOVA tests. Statistical significance was set at P < .05.
The number of CCs required for each patient followed the protocol established by the Pediatric Cardiology Unit. This protocol mandates EMB at specific time points: 10 to 14 days after HT, at 1, 3, 6, 12, and 24 months post-HT, and subsequently every 2 years. Additional CCs are performed if rejection is suspected. If rejection is confirmed (grades ≥ 2 cellular and ≥ 1 humoral), a follow-up EMB is performed 2 weeks later, following appropriate treatment. The EMB samples are obtained from the right side of the interventricular septum using a 6-French bioptome, via the right jugular vein. Coronary angiography is routinely performed in the first 3 to 6 months, and subsequently every 2 years together with EMB. Coronary intravascular ultrasound (IVUS) is carried out in the anterior descending artery and is associated with coronary angiography in patients weighing > 20 kg. Pathological findings are defined as intimal layer measurements ≥ 0.5 mm.
RESULTS
Demographic and heart transplant data
A total of 61 participants were included, of whom 37 (60.66 %) were boys. The underlying disease was CHD in 22 patients (36.07%) and cardiomyopathy in 39 (64.93%). Five participants (8.20%) were categorized as having hypoplastic left heart syndrome (HLHS). All patients with CHD had undergone at least 1 cardiac surgical intervention before HT.
The mean age at HT was 96.24 ± 89.47 months, with no differences between groups. A higher proportion of the CHD group required extracorporeal life support (ECLS) after HT than the cardiomyopathy group (40.00% vs 10.26%; P = .005) and had longer admissions to the pediatric intensive care unit (PICU). In both groups, New York Heart Association functional class before HT remained between grades 3 and 4.
Dividing the study years in time periods (2002-2015, 2016-2021) revealed that the number of HT recipients increased over the years. Although cardiomyopathy was the most common underlying disorder in all time groups, patients with CHD showed a nonstatistically significant increase, representing 22.23% of patients in the first period, and 35.71% in the final 5-year period (P = .722). See table 1 and figure 1 for further demographic and transplant data.
Table 1. Demographic and heart transplant data
| Demographic and heart transplant variables | Total | Cardiomyopathy | Congenital heart disease | P |
|---|---|---|---|---|
| Number of patients | 61 | 39 | 22 | |
| Male sex | 37 (60.66) | 23 (58.97) | 14 (63.64) | .403 |
| Patients with 1 previous cardiac surgical intervention | 27 (44.26) | 25 (64.10) | 2 (10) | .029* |
| Patients with 2 previous cardiac surgical interventions | 18 (29.51) | 0 (0) | 18 (81.81) | < .001* |
| Univentricular physiology | 10 (16.39) | 0 (0) | 10 (50) | .000* |
| Hypoplastic left heart syndrome | 5 (8.20) | 0 (0) | 5 (22.73) | .02* |
| Patients with cyanosis | 9 (14.75) | 0 (0) | 9 (45) | .000* |
| Pulmonary hypertension | 17 (27.87) | 7 (17.95) | 10 (50) | .377 |
| Number of treatments for pulmonary hypertension | 0 (0) | 0 (0) | 1.5 (0-4) | .075 |
| Age (months) at transplant | 74.00 (20.00-168.00) | 72.00 (20.00-127.00) | 77.50 (24.25-169.50) | .440 |
| Weight (kg) at transplant | 31 (11.80-45.00) | 22.00 (10.85-40.50) | 35.00 (17.50-56.00) | .067 |
| Patients requiring posttransplant ECLS | 12 (19.67) | 4 (10.26) | 8 (40) | .005* |
| PICU days after transplant | 15.5 (10.75-30) | 14.00 (9.75-24.50) | 26.50 (12.75-76.25) | .035* |
ECLS, extracorporeal life support; IQR, interquartile range; PHT, pulmonary hypertension; PICU, pediatric intensive care unit. Qualitative data are expressed as absolute number and percentage and quantitative variables as the median and interquartile range. * Statistical significance for the student t or chi-square tests. | ||||
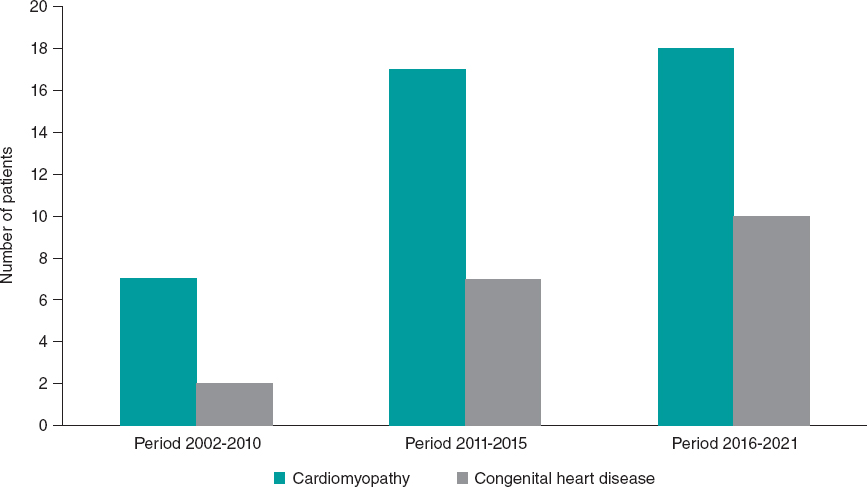
Figure 1. Distribution of underlying heart disease over time periods.
Cardiac catheterizations performed prior to heart transplant
The number of patients with at least 1 CC did not differ between the CHD and cardiomyopathy groups (P = .07). However, the mean number of previous CCs was significantly higher in patients with CHD (P = .014). CC was mainly performed for diagnostic purposes in the cardiomyopathy group (45.00%), followed by EMB (16.70%) and by atrioseptostomy with stent for left cavity unloading in ECLS (15%). There were 8 coronary angiograms and 1 IVUS, as well as 2 coronary balloon angioplasties and 3 coronary angioplasties with stent, in OM1 and circumflex arteries. The coronary angiography-related procedures were performed in a single patient, who had previously undergone HT.
In the CHD group, CCs were mostly performed for diagnostic purposes, accounting for 57.30% of the procedures, followed by major aortopulmonary collateral closure (11.0% of the activity), and by pulmonary artery angioplasty with stent (10.26%).
A higher percentage of diagnostic procedures were unrelated to rejection surveillance in the CHD group but this difference was not statistically significant. Nonetheless, there was a significantly higher predominance of rejection surveillance in the cardiomyopathy group (57.3% CHD vs 75.0% cardiomyopathy).
When we divided the study in 2 time periods, 2002 to 2014 and 2015 to 2021, there was a median of 0 [IQR, 0-2] procedures per person in the first period, and 2 [IQR, 0-3.25] in the recent period, showing a tendency to nonsignificant growth in activity (see table 2 for further information).
Table 2. Data on pretransplant cardiac catheterizations
| Pretransplant procedures | Cardiomyopathy (n = 39) | Congenital heart disease (n = 22) | P |
|---|---|---|---|
| Patients with previous CC | 17 (43.59%) | 18 (81.82%) | .282* |
| 1 CC | 12 | 6 | 0 |
| 2 CC | 2 | 1 | 0 |
| 3 CC | 2 | 5 | 0 |
| 4 CC | 1 | 1 | 0 |
| 5 or more CC | 0 | 5 | 0 |
| CC per person; median (IQR) | 0 (0-1) | 2.5 (1-3.75) | .014* |
| Number of previous procedures | 60 | 82 | 0 |
| Number of therapeutic interventional procedures (n) | 15 | 35 | 0 |
| Balloon atrioseptostomy | 2 | 0 | 0 |
| Atrioseptostomy with stent | 5 | 3 | 0 |
| Coronary angioplasty with stent | 3 | 0 | 0 |
| Interatrial stent redilatation | 2 | 0 | 0 |
| Balloon coronary angioplasty | 2 | 0 | 0 |
| IVUS | 1 | 0 | 0 |
| Collateral artery closure | 0 | 9 | 0 |
| Pulmonary branch angioplasty with stent | 0 | 9 | 0 |
| Cavopulmonary anastomosis balloon angioplasty | 0 | 2 | 0 |
| Cavopulmonary anastomosis angioplasty with stent | 0 | 1 | 0 |
| Aortic valvuloplasty | 0 | 3 | 0 |
| Ventricular septal defect closure | 0 | 2 | 0 |
| Coronary fistula embolization | 0 | 2 | 0 |
| Pulmonary trunk angioplasty with stent | 0 | 1 | 0 |
| Superior cava vein balloon angioplasty | 0 | 1 | 0 |
| Iliac stent dilatation (previous migration) | 0 | 1 | 0 |
| Fontan fenestration (failure) | 0 | 1 | 0 |
| Diagnostic procedures (percentage of total procedures) | 45 (75.0%) | 47 (57.3%) | .029* |
| Coronary angiography | 8 | 4 | 0 |
| Endomyocardial biopsy | 10 | 0 | 0 |
| Diagnostic catheterization | 27 (45.0%) | 43 (52.4%) | .380 |
CC, cardiac catheterization; ECLS, extracorporeal life support; IVUS, intravascular ultrasound. Qualitative data are expressed as absolute numbers and percentages and quantitative variables as median the and interquartile range. *Statistical significance. | |||
Postheart transplant surgery cardiac catheterization
After the HT surgery, a total of 389 CC were obtained, corresponding to 608 procedures. The mean number of procedures per CC was 1.37 ± 0.83. The mean number of CCs per person was 6.71 ± 4.13.
The median number of procedures per person was 13 [IQR, 9-17] in the first period (2002-2015), which decreases to 8 [IQR, 2-9.25] in the second period (2016-2021), given the shorter follow-up time in the latter period.
Rejection surveillance
Most CCs were performed for rejection surveillance: EMBs represented 63.10% of post-HT activity, coronary angiograms up to 18.29%, and IVUS 11.53%. The proportion of rejection surveillance studies was significantly higher in patients with cardiomyopathy than in those with CHD. EMB was positive in 7.39% of cases for cellular rejection, and in 3.17% for humoral rejection. Up to 9.40% of EMB were follow-up EMBs secondary to rejection found in a previous CC.
CAV was diagnosed in 6.71% of coronary angiograms. The most frequently involved coronary artery was the anterior descending artery, found in the 36% of positive studies, followed by the left ostium, circumflex and ramus intermedius, with 14% each. No differences were found between baseline heart disease groups, with cardiomyopathy having a positivity index of 29.07% and CHD 16.67%.
Among patients undergoing IVUS (n = 70), 31.43% met the criteria for positivity.
No differences were found in the positivity index depending on the baseline heart disease, with 29.07% in the cardiomyopathy group and 13.33% in the CHD group being positive.
The mean time to CAV diagnosis was 37.1 [IQR, 13-47.5] months after HT, corresponding to the fourth to 13th CC.
Interventional procedures
The most common techniques were superior cava vein and pulmonary artery balloon angioplasties, each representing 20.45% of the interventional procedures and corresponding to 18.03% (n = 11) of the patients for the superior cava vein and to 4 patients for the pulmonary arteries.
Cava vein angioplasty was performed at a median time of 2.5 [IQR, 0.75-6] months, and 6 (40%) of the procedures took place within the first 2 months after HT.
Diagnosis of stenosis was secondary to clinical symptoms in the superior cava vein in 2 patients and pericardial effusion in 1 patient. In 1 patient, signs of congestive hepatopathy led to the diagnosis of inferior cava vein stenosis. The remaining indications were driven by echocardiographic findings or observation during biopsy. Patients who underwent superior cava vein angioplasty, either with a balloon or with a stent, showed a tendency to lower mean age (63.6 vs 90.6 months) and weight at HT and higher discrepancies in weight ratios, but without statistically significant differences (P values .233, .243 and .605, respectively). This group did not have a higher number of previous surgical interventions (P = .460) or higher ECLS requirements (P = .253). We did not observe a higher proportion of patients with CHD in the cava stenosis group (P = .221). Three patients underwent more than 1 angioplasty due to restenosis.
Pulmonary angioplasty, either with a balloon or stent, was required mainly at the pulmonary branches. The median time from transplant to pulmonary angioplasty was 4 [IQR, 2-26] months, and 3 of them were performed during the first 2 months after surgery.
Coronary treatments were required only twice, one consisting of angioplasty with stent implantation, and the other in a thrombolysis.
Pulmonary artery angioplasty, whether with balloon (P < .001) or stent (P = .011), superior cava vein angioplasty with stent (P = .038), and right ventricle-to-pulmonary artery tube angioplasty (P = .037) were more frequent in CHD patients (see table 3 and figure 2 for a detailed description).
Table 3. Posttransplant activity and comparison between underlying disease groups
| Postheart transplant procedures | Total | Cardiomyopathy | Congenital heart disease | P |
|---|---|---|---|---|
| Superior cava vein balloon angioplasty | 9 | 4 | 5 | .125 |
| Pulmonary artery balloon angioplasty | 9 | 0 | 9 | <.001* |
| Pulmonary resistance study | 6 | 2 | 4 | .066 |
| Inferior cava vein balloon angioplasty | 3 | 2 | 1 | .957 |
| Pulmonary artery angioplasty with stent | 3 | 0 | 3 | .011* |
| Cava vein thrombi-related procedures | 3 | 1 | 2 | .195 |
| Innominate vein angioplasty | 2 | 1 | 1 | .582 |
| Superior cava vein angioplasty with stent | 2 | 0 | 2 | .038* |
| Right ventricle-pulmonary artery conduit balloon angioplasty | 2 | 0 | 2 | .038* |
| Inferior cava vein angioplasty with stent | 1 | 1 | 0 | .493 |
| Coronary angioplasty with stent | 1 | 1 | 0 | .493 |
| Marginal coronary artery thrombolysis | 1 | 1 | 0 | .493 |
| Pericardiocentesis | 1 | 1 | 0 | .493 |
| Total | 44 | 14 | 29 | |
| Rejection surveillance activity | ||||
| Endomyocardial biopsy | 383 | 255 | 128 | .274 |
| Coronary angiography | 111 | 89 | 22 | .001* |
| IVUS | 70 | 55 | 15 | .046* |
| TOTAL | 564 | 399 | 164 | |
CC, cardiac catheterization; ECLS, extracorporeal life support; IVUS, intravascular ultrasound. Qualitative data are expressed as absolute numbers and percentages and quantitative variables as the median and interquartile range. * Statistical significance. | ||||
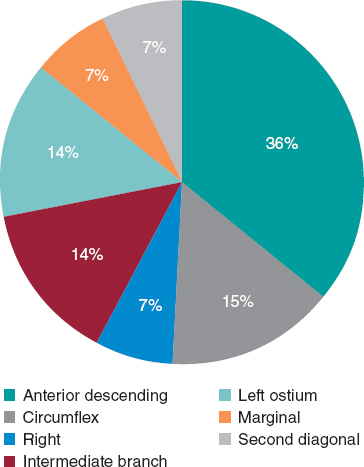
Figure 2. Distribution of the artery affected in coronary angiography.
Generalities
The mean CC duration was 64.65 ± 38.02 minutes. When participants were divided into 2 periods (2002-2015 and 2016-2021), there were a significantly (P < .001) higher number of procedures per person in the first period, with a mean of 12.67 ± 7.55, than in the second period, with a mean of 6.54 ± 4.05.
Complications, both systemic and local and of all degrees severity, occurred in 2.80% of the total number of CCs. Systemic complications consisted of 1 ST-segment depression at initiation of the procedure that spontaneously disappeared, atrial flutter unresponsive to atrial overstimulation but that reverted with electrical cardioversion, 1 bronchospasm with anesthetic induction requiring PICU admission for elective extubation (performed after 24 hours), 2 right coronary spasms that reverted with nitroglycerine, a second and a third degree temporary atrioventricular blockage, which required a dose of epinephrine, 1 pulmonary hypertension crisis treated effectively in the catheterization laboratory, 1 moderate tricuspid valve regurgitation, and 1 posterior reversible encephalopathy syndrome. Local complications consisted of 1 puncture site hematoma and 1 femoral artery vasospasm.
Distribution according to time period revealed that the complication rate was 1.91% in the first period (2002-2015) and 1.64% in the second (2016-2021). ECLS-supported patients corresponded to 1.60% of the activity. In the first 6 months after HT, ECLS was being used in 47.56% of procedures (figure 3).
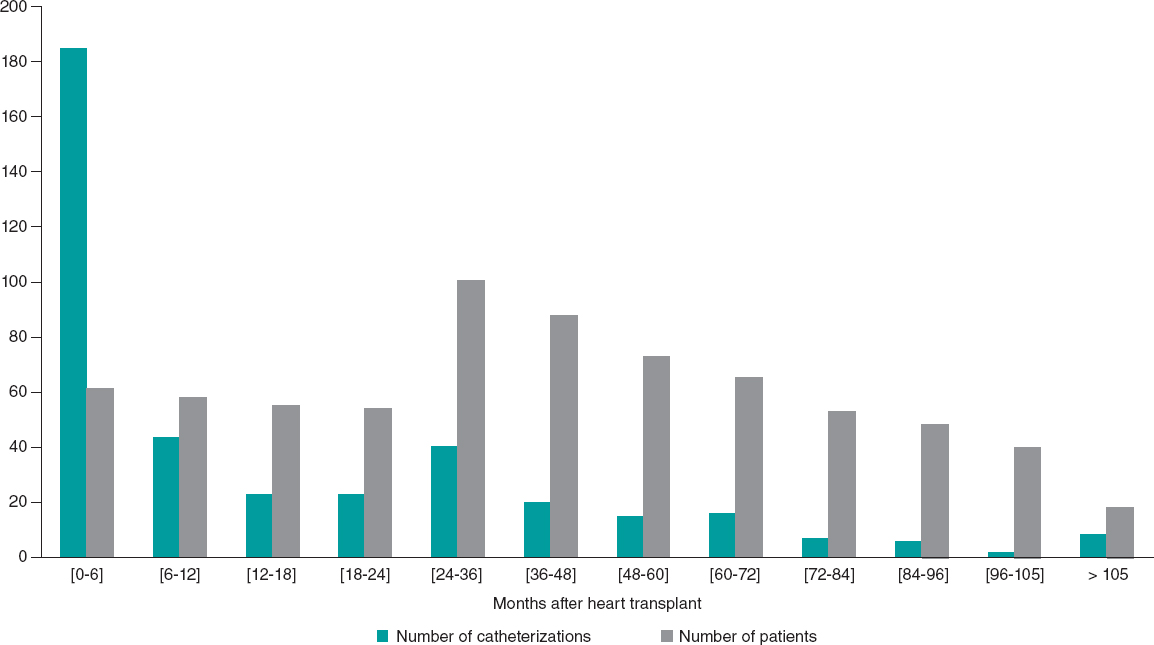
Figure 3. Variation in frequency of cardiac catheterization according to time from transplant and number of patients at follow-up.
The mean patient follow-up was 6.48 ± 4.07 years. Survival at this point was 88.52%. No significant associations were found in the analysis of risk factors for mortality, in which we assessed the number of CCs before HT, the number of therapeutic procedures, the total number of CCs post-HT, and the number of patients who had undergone superior cava vein or pulmonary artery angioplasty.
Analysis of risk factors for greater requirement of procedures
In the analysis of factors associated with a higher number of CC procedures after HT, we found no association with the number of previous interventional procedures (P = .149) or CC (P = .059), or with having undergone at least 1 prior CC (P = .107). The subgroup with univentricular CHD required a significantly higher number of CCs after HT (P = .03). Weight and age at HT were not significantly associated with the need for subsequent CC. A greater donor- recipient weight discrepancy was not found to be a predictive factor. A higher need for interventional procedures was not associated with length of PICU stay, the number of days under mechanical ventilation, or a medical history of pulmonary hypertension or renal failure. Longer follow-up was associated with a larger number of CC procedures due to the longer amount of time studied (table 4).
Table 4. Study of factors associated with requirement for catheterization procedures after heart transplant
| Variable | Test result | P |
|---|---|---|
| Type of heart disease | Mann-Whitney U test = 555.5 | .142 |
| Univentricular physiology | Kruskal Wallis test = 4.71 | .030* |
| Previous surgery | Kruskal Wallis test = 2.52 | .284 |
| Pulmonary hypertension prior to HT | U Mann Whitney test = 281 | .134 |
| Number of pulmonary hypertension treatments | R2 = -0.186; 95%CI, -0.419 - 0.069 | .150 |
| Number of previous cardiac catheterizations | R2 = - 0.233; 95%CI, -0.460- 0.220 | .073 |
| Number of previous interventional procedures | R2 = -0.19; 95%CI, -0.426 - 0.069 | .149 |
| Renal failure prior to HT | U Mann Whitney Test = 358 | .755 |
| Age at HT | R2 = - 0.001; 95%CI, -0.388 - 0.109 | .992 |
| Weight at HT | R2 = - 0.072; 95%CI, -0.183 - 0.318 | .583 |
| Weight differences (ratio between donor-recipient) | R2 = 0.0164; 95%CI, -5.39 - 1.93 | .347 |
| Length of stay in the pediatric intensive care unit | R2 = 0.038; 95%CI, -0.249 - 0.319 | .798 |
| Mechanical ventilation days | R2 = 0.140; 95%CI, -0.325 - 0.255 | .348 |
| Length of follow-up | R2 = 0.394; 95%CI, 0.158 - 0.588 | .002* |
CI, confidence interval; HT, heart transplant; R2, Pearson correlation. * Statistical significance. | ||
DISCUSSION
In this study, we evaluated pediatric HT-related CC activity in a university tertiary hospital. There were 61 patients and 607 procedures. Most post-HT activity was performed for rejection surveillance, representing up to 92.75% of procedures. The most frequently performed procedure was EMB, followed by coronary angiography and intravascular ultrasound, which allowed diagnosis of chronic allograft vasculopathy (figure 4).
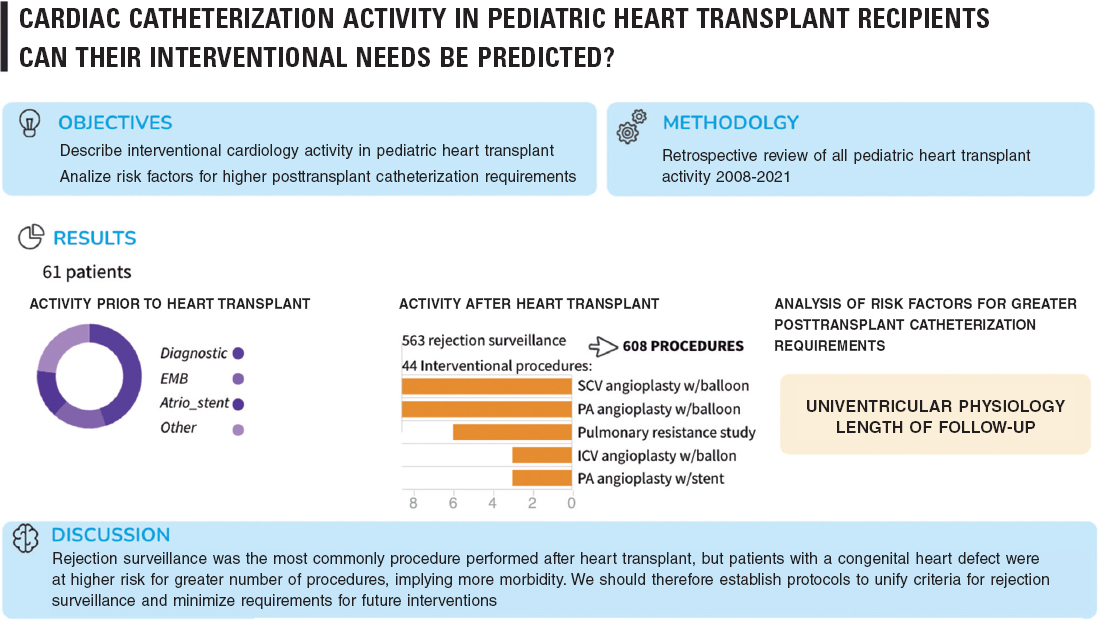
Figure 4. Central illustration. Key points of the article. Atrio_stent, interatrial stent; EMB, endomyocardial biopsy; ICV, inferior cava vein; PA, pulmonary arteries; SCV, superior cava vein.
This distribution is in line with other publications. A multicenter study including almost 50 000 pediatric catheterization procedures in Philadelphia found that the most frequent HT-related diagnostic techniques were CCs, including EMB.10
IVUS clearly showed higher sensitivity for detecting coronary allograph vasculopathy, which is unsurprising as the technique allows a more detailed diagnosis than the imaging provided by coronary angiography. In our study, positive results were obtained in 6.71% of coronary angiograms vs 31.43% of IVUS studies. Therefore, IVUS provides a much earlier diagnosis and implementation of measures.
Rejection surveillance represented a statistically higher relative percentage of activity in patients with cardiomyopathy. This was because CHD participants also required other types of procedures secondary to previous surgical interventions or to the sequelae of the CHD itself. An example could be the underdevelopment of pulmonary branches commonly found in cyanotic or univentricular patients with previous banding or systemic-pulmonary fistulae, which cannot be replaced in the HT surgery, or which are left untouched during the HT to shorten the surgical time and are delayed for a percutaneous approach.
Our protocol, described in the Methods section, is similar to the protocols of other centers, such as that of the Helsinki University Hospital, where they proceed to EMB at 1- to 2-weeks during the first 4 to 6 postoperative weeks in children aged >24 months. Once the patient is discharged, EMB is performed at 3, 6, 12, 18 months and after that, on a yearly basis until the patient reaches adulthood. Coronary angiography is performed annually.11 Although a third of their IVUS were positive for CAV, only 1 patient required coronary stenting. These results differ from data in adults, whose mature immune system plays a detrimental role.12
Regarding interventional catheterizations, more than half of therapeutic procedures were due to anastomosis stenosis, mainly of the superior cava vein and pulmonary arteries, predominantly at the level of the branches followed by the common trunk. Less frequently performed were inferior cava vein angioplasties and coronary treatments, including stenting and mechanical thrombectomy.
In our study, the percentage of superior cava vein obstruction accounted for 13.11% of the patients. Salavitabar et al.5 reported a prevalence of superior cava vein obstruction in HT of 3.4%, diagnosed either clinically secondary to superior cava vein syndrome or chylous effusion, or by echocardiography. All of the patients underwent angioplasty in the first 10 months after surgery, and as many as two-thirds were performed within the first 2 months. Statistically significant risk factors for superior cava vein stenting included younger age, lower recipient weight, a history of congenital heart disease, and previous superior cavopulmonary anastomosis. In our cohort, we did not obtain statistically differences in these factors in the superior cava vein stenosis group. The authors propose measuring the pressure at the right atrium and high superior cava vein (SCV) in routine EMB in patients with these risk characteristics. In our center, right heart cavity pressures are measured in routine EMB. No cases of stent thrombosis were diagnosed in the study by Salavitar et al.5 Their protocol includes enoxaparin for 3 to 6 months if a stent is placed. Sachdeva et al.13 reported 5.1% of stented superior cava vein obstructions in a pediatric HT cohort, in contrast with our 13.11%. The median age at HT in these 7 patients was lower than in our cohort (9 vs 63.6 months) and their median weight was also lower (8.7 vs 10.5 kg). Despite lower rates of superior cava vein obstruction than in our cohort, the median follow-up was shorter (48 months) than in our study (see figure 4 for a summary).
Tadros et al.14 reported an incidence of 30% of superior cava vein stenosis, of which almost 6% had to undergo intervention. Risk factors for its development were smaller weight and younger age, as well as previous cavopulmonary surgery or cava procedures.
A probable explanation for the higher proportion of superior cava vein stenosis in our study than in other reports in the literature could be the complex anatomy of most of our HT recipients, who had congenital heart disease and several previous surgical interventions, as well as anomalous venous anatomies in some cases. The initial choice between performing a bare angioplasty or implanting a stent in pediatric patients is influenced by multiple factors, rather than solely by aiming for the best final procedural outcome. These factors include the small patient and stent sizes, the need for multiple consecutive balloon dilations to accommodate the patient’s growth, the high risk of pulmonary leaflet entrapment during stent implantation in the pulmonary trunk, the strong possibility of future new HTs, and the goal of avoiding stent placement in sutures, among other considerations. These factors may lead to a modification of the initial stent implantation strategy in favour of a bare angioplasty approach, if the patient’s anatomy is suitable, and when there is the possibility of achieving a sufficiently good final result.
Pulmonary stenosis represented 27.27% of the interventional therapeutic activity in this work, corresponding to 4 patients (6.55%). This percentage is higher than that in the single article found in the literature, which described rates of 1.44%.2 A probable explanation for this discrepancy is that the present study included a higher number of patients with congenital heart disease.
Coronary interventions accounted for a few cases, as reported in other studies, such as the American and Canadian Pediatric Heart Transplant Study, in which revascularization was required in 0.90% of their patients.15
To our knowledge, this is the first study to analyze the predisposing and associated factors for a higher number of catheterizations after HT. The antecedent of univentricular physiology was statistically significant, while, in contrast, the number of interventional procedures or catheterizations prior to HT was not. No differences were found depending on patient age or weight at HT. Worse baseline status, such as patients with pulmonary hypertension or renal failure, or perioperative complications, such as longer PICU stay or mechanical ventilation support, were not significantly associated in our study. We did not perform a multivariate analysis due to the lack of significant univariate variables.
HLHS is known to worsen the outcome of HT, as reflected in our study.2 However, a publication by Miyamoto et al.3 found no increased risk for pulmonary angioplasty in HLHS patients who had undergone bilateral pulmonary banding.
Limitations
The limitations of the study are those inherent to retrospective studies, although a rigorous and extensive database of the CCs performed exists in our center. Another limitation is that the study was performed in a single center and it would be advisable to obtain data in a multicenter study. Extending the follow-up time would have allowed wider and more accurate interpretation of the results, particularly regarding coronary allograph vasculopathy outcomes. In addition, further studies are needed on the risk factors than can increase the requirements for post-HT CCs. The retrospective nature of this work in a pediatric population means that gender was assigned according to that given at birth and documented in the medical history; hence we were unable to comply with the SAGER guidelines.
CONCLUSIONS
Although pediatric HT recipients have long-life expectancy, they considerable morbidity due to interventional procedures, mainly performed for rejection surveillance. Despite only finding statistical significance in univentricular physiology as an associated factor for a higher number of post-HT catheterizations, there was a tendency indicating that previous interventions and smaller patients are at higher risk.
Multicenter studies with a high volume of patients and long follow-up are needed to establish follow-up protocols for these patients.
FUNDING
No conflicts of interests or financial support are declared.
ETHICAL CONSIDERATIONS
This work was approved by the local ethics committee, with a waiver of informed consent form due to its retrospective methodology. The principles of the Declaration of Helsinki were followed throughout the study. Despite the authors agreeing with the SAGER guidelines, because of the retrospective nature of this work and its performance in a pediatric population, gender was assigned according to the sex assigned at birth and documented in the medical history.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence has been used in the preparation of this work.
AUTHORS’ CONTRIBUTIONS
A. Freixa-Benavente performed the data recollection, analysis of results and manuscript writing. P. Dolader and F. Gran participated in the conception of the study, in providing guidance, and in reviewing the manuscript. P. Betrián-Blasco led the project, made the initial proposal, conceived the original hypothesis, led the research, and reviewed and approved the manuscript and analysis.
CONFLICTS OF INTEREST
P. Betrián conducts personal advisory tasks on devices for Occlutech, not related to this work. No other conflicts of interest are declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Pediatric HT recipients undergo a high number of CCs, involving multiple hospital admissions and significant morbidity.
- However, because HT is a rare procedure, there are a scarcity of protocols and information on how to perform rejection surveillance, which is the most common activity in the catheterization laboratory.
WHAT DOES THIS STUDY ADD?
- We provide a detailed analysis of the activity of the pediatric interventional cardiology unit in a tertiary center. Globally, the most frequently performed procedures were diagnostic catheterizations for rejection surveillance. The most frequent therapeutic interventional techniques were superior cava vein and pulmonary artery balloon angioplasties.
- Patients with univentricular physiology had a higher need for post-HT CCs, but no differences were found for other congenital diseases, age, weight, or longer intensive care unit admissions.
- There is a need to unify protocols across multiple centers.
REFERENCES
1. Chen S, Dykes JC, McElhinney DB, et al. Haemodynamic profiles of children with end-stage heart failure. Eur Heart J. 2017;38:2900-2909.
2. Morchi GS, Pietra B, Boucek MM, et Chan KC. Interventional cardiac catheterization procedures in pediatric cardiac transplant patients:Transplant surgery is not the end of the road. Catheter Cardiovasc Interventions. 2008;72:831-836.
3. Miyamoto SD, Pietra BA, Chan KC, et al. Long-term outcome of palliation with internal pulmonary artery bands after primary heart transplantation for hypoplastic left heart syndrome. Pediatr Cardiol. 2009;30:419-425.
4. Jeewa A, Chin C, Pahl E, et al. Outcomes after percutaneous coronary artery revascularization procedures for cardiac allograft vasculopathy in pediatric heart transplant recipients:A multi-institutional study. J Heart Lung Transplant. 2015;34:1163-1168.
5. Dipchand AI. Current state of pediatric cardiac transplantation. Ann Cardiothorac Surg. 2018;7:31-55.
6. Daly KP, Marshall AC, Vincent JA, et al. Endomyocardial biopsy and selective coronary angiography are low-risk procedures in pediatric heart transplant recipients:Results of a multicenter experience. J Heart Lung Transplant. 2012;31:398-409.
7. Salavitabar A, Flyer JN, Torres AJ, et al. Transcatheter stenting of superior vena cava obstruction after pediatric heart transplantation:A single-center experience assessing risk factors and outcomes. Pediatr Transplant. 2018;22:e13267.
8. Lim HS, Hsich E, Shah KB. International Society of Heart and Lung Transplantation position statement on the role of right heart catheterization in the management of heart transplant recipients. J Heart Lung Transplant. 2019;38:235-238.
9. Molkentin JP, Nägele MP, Frank M, et al. Prognostic value of mean pulmonary artery pressure in the stable phase after heart transplantation. Eur J Cardiothorac Surg. 2017;52:775-780.
10. O'Byrne ML, Glatz AC, Faerber JA, et al. Interhospital Variation in the Costs of Pediatric/Congenital Cardiac Catheterization Laboratory Procedures:Analysis of Data From the Pediatric Health Information Systems Database. J Am Heart Assoc. 2019;8:e011543.
11. Raissadati A, Pihkala J, Jahnukainen T, Jokinen E, Jalanko H, Sairanen H. Late outcome after paediatric heart transplantation in Finland. Interact Cardiovasc Thorac Surg. 2016;23:18-25.
12. Wellnheofer E, Lehmkuhl H, Hiemann N, et al. Monocenter study of percutaneous coronary interventions in 154 cardiac transplant recipients with chronic allograft vasculopathy. J Heart Lung Transplant. 2007;26:182-183.
13. Sachdeva R, Seib PM, Burns SA, Fontenot EE, Frazier EA. Stenting for superior vena cava obstruction in pediatric heart transplant recipients. Catheter Cardiovasc Interv. 2007;70:888-892.
14. Tadros HJ, Whelihan JT, Lopez-Colon D, et al. Risk factors associated with post-transplant superior caval vein stenosis in paediatric heart transplantation. Cardiol Young. 2021;31:1589-1594.
15. Jeewa A, Chin C, Pahl E, et al. Outcomes after percutaneous coronary artery revascularization procedures for cardiac allograft vasculopathy in pediatric heart transplant recipients:A multi-institutional study. J Heart Lung Transplant. 2015;34:1163-1168.











