ABSTRACT
Introduction and objectives: Coronary computed tomography angiography (CCTA) has become the gold standard to measure the size of the aortic annulus and better select the size of transcatheter heart valves (THV) in patients undergoing transcatheter aortic valve implantation (TAVI). However, in selected cases, CCTA may not be feasible. Angiographic aortic annulus (AAA) measurements during TAVI may be an alternative and should be evaluated for precision regarding the proper selection of THV sizes. We sought to investigate the correlation between AAA and CCTA measurements for the proper selection of balloon-expandable valve (BEV) sizes in patients undergoing TAVI.
Methods: Patients undergoing TAVI with BEV and high-quality CCTA were included. AAA measurements were obtained in the standard 3-cusp view after aortic root aortography. Angiographic distance between non- and left coronary cusps were compared to CCTA annulus measurements. Endpoints were diagnostic tests and correlations between angiographic and CCTA measurements, and the composite endpoint of the VARC-3-defined efficacy (technical success, correct position, and intended performance), and safety profile (multiple valves, valve embolization, pacemaker implantation, and more than moderate valvular regurgitation).
Results: Regarding the Sapien family of THV, aortography-based distance measurements showed a correlation of 0.528 (P < .01), 0.451 (P < .01), and 0.579 (P < .01) for 23 mm, 26 mm, and 29 mm valves with CCTA-based distance measurements. No difference was seen regarding the VARC-3-defined efficacy (94.2% vs 96.0%; P = .60) and safety profile (90.9% vs 91.9%; P = .84) among cases showing discordant and concordant pairs of measurements.
Conclusions: AAA measurement showed a moderate diagnostic test and Spearman’s correlation coefficient compared to CCTA-based annulus assessment for perioperative THV size selection. This strategy could potentially enable TAVI in patients in whom access to preoperative CCTA is not available.
Keywords: Non-coronary cusp. Left coronary cusp. Aortography. Angiographic aortic annulus measurements. Transcatheter aortic valve implantation.
RESUMEN
Introducción y objetivos: La angiografía por tomografía computarizada (angio-TC) es el estándar para medir el anillo aórtico en pacientes tratados mediante implante percutáneo de válvula aórtica (TAVI), aunque en algunos casos podría no ser factible. Debería evaluarse la precisión de las medición del anillo aórtico angiográfica (AAA) durante el TAVI como alternativa para elegir el tamaño correcto de la válvula cardiaca percutánea. Por ello, investigamos la correlación entre las mediciones angiográficas y por angio-TC para elegir el tamaño adecuado de la válvula en pacientes que reciben un TAVI.
Métodos: Se incluyeron pacientes de TAVI con prótesis de balón expandible y angio-TC de alta calidad. Las mediciones del AAA se obtuvieron de la angiografía de la raíz aórtica en proyección de 3 cúspides. Se comparó la distancia angiográfica entre la cúspide izquierda y no coronariana con las mediciones de angio-TC. Se evaluaron la prueba diagnóstica y la correlación entre las medidas angiográficas y de angio-TC, así como la eficacia (éxito técnico, posición correcta y desempeño intencionado) y la seguridad (múltiples válvulas, embolización, implante de marcapasos e insuficiencia valvular moderada o mayor) definida por VARC-3.
Resultados: Para válvulas con balón expandible de 23 mm, la distancia en la aortografía tuvo una correlación de 0,528 (p < 0,01) comparada con las mediciones de angio-TC; para las de 26 mm, la correlación fue de 0,451 (< 0,01), y para las de 29 mm fue de 0,579 (< 0,01). No hubo diferencia en cuanto a eficacia (94,2 frente a 96,0%; p = 0,60) y seguridad (90,9 frente a 91,9%; p = 0,84) entre casos con medidas concordantes y discordantes.
Conclusiones: Las mediciones del AAA mostraron un moderado valor de prueba diagnóstica y de correlación Spearman en comparación con la angio-TC para elegir el tamaño de la válvula cardiaca percutánea. Esta estrategia podría permitir un TAVI en situaciones en que la angio-TC no esté disponible.
Palabras clave: Cúspide no coronariana. Cúspide coronaria izquierda. Aortografía. Mediciones angiográficas del anillo aórtico. Implante percutáneo de válvula aórtica.
Abbreviations
BEV: balloon-expandable valve. CCTA: coronary computed tomography angiography. LCC: left coronary cusp. NCC: non-coronary cusp. TAVI: transcatheter aortic valve implantation. THV: transcatheter heart valve.
INTRODUCTION
During transcatheter aortic valve implantation (TAVI), coronary computed tomography angiography (CCTA) remains the key factor to determine the characteristics of the aortic valve and predefine the size of annular valve prior to the selection of specific transcatheter heart valves (THV).1,2 Several CCTA protocols were described to achieve reproducible and reliable aortic annulus measurements.3,4 At the same time, transthoracic and transesophageal echocardiographic measurements were used to determine the aortic valve annular size showing good correlation with the gold standard of direct surgical and CCTA-based measurements.5,6 However, CCTA showed better image quality acquisition, detailed evaluation of the aortic annulus, and other useful anatomies for transfemoral TAVI (aorto-iliac-femoral vessels)7 making CCTA the default strategy for preoperative planning.
Adequate THV size selection is an important factor to prevent patient-prosthesis mismatch and reduce the risk of over- and under-sizing and, hence, the increased risk of all cause-mortality and unplanned repeat reintervention.6,8,9 While CCTA has been established as the gold standard method for annular sizing pre-TAVI implantation,4 an associated risk between radiation exposure and cancer, and contrast media and nephropathy has also been described.10,11 Furthermore, in selected cases, CCTA may not be feasible prior to TAVI following emergency clinical indications and/or the patients’ unstable conditions.
Aortography-only annular measurement was described as an efficient technique to determine the size of aortic annulus and select the size of the THV.12,13 Based on the standard 3-cusp view, the angiographic determination of anatomical dimensions with contrast media (and/or balloon-sizing) can facilitate the identification of proper annular size when CCTA-based sizing is not available.14-17 Aortography-based measurements have been shown to correlate with direct anatomical preoperative aortic annulus measurements.13
Against this background, we sought to investigate whether angiographic aortic valve annular measurements between the non-coronary (NCC) and left coronary cusp (LCC) correlate with CCTA-based measurements to facilitate proper size selection of the THV in a retrospective, all-comers, single-center cohort of patients undergoing TAVI.
METHODS
Study population
This retrospective, observational analysis evaluated all consecutive patients undergoing transfemoral TAVI following heart team evaluation at the German Heart Center cardiovascular disease unit in Munich, Germany. Transfemoral TAVI was performed using a minimalistic approach18 in all cases, while THV size selection was left to the operator’s discretion based on size chart, CCTA measurements, anatomical factors including calcium distribution and severity, aortic valve annular size, coronary height, and disease.
All patients with native tricuspid calcified aortic valve disease, and available high-quality CCTA for TAVI were included in this study. Procedural information was obtained from a customized database and screened for all patients treated from January 2014 through December 2021 at the German Heart Center in Munich, Germany. During this period, a total of 2500 transfemoral TAVI cases were performed using commercially available balloon-expandable (1865) and self-expanding (635) THVs. Only those who received the SAPIEN 3 or the SAPIEN 3 Ultra (Edwards Lifesciences, United States) balloon-expandable valves (BEV) were included in this analysis.
The study was performed in full compliance with the principles set forth in the Declaration of Helsinki, and all patients gave their written informed consent to undergo the procedure. Ethical approval was obtained from the Technical University of Munich ethical committee under registry no. OBSERVTAVI (#525/17). CCTA measurements were performed before THV implantation. Angiographic aortic valve annular measurements between the NCC and LCC were performed offline and documented in the database. The baseline clinical and procedural characteristics (including size of the implanted THV and angiographic aortic regurgitation after implantation), and test lab results were obtained from registry data or the clinical records, as appropriate. Regarding the Valve Academic Research Consortium 3 (VARC-3) defined safety and efficacy profile, in-hospital and discharge follow-up was monitored and registered. A 30-day follow-up was established via telephone call, hospital visits or follow-up letter.
Coronary computed tomography angiography measurements
CCTA was analyzed by 1 experienced cardiologist (HA) while a second experienced cardiologist (JM) analyzed a sample of 40 cases to determine inter-observer variability. Using multi-slice computed tomography reconstruction, quantitative measures of the aortic valve annular size (minimum, maximum and mean diameter, perimeter, and area) were obtained based on predefined protocols2 using 3-Mensio software (Pie Medical Imaging, The Netherlands). In summary, the 3 hinge points of the aortic cusps were detected and selected. After proper identification of the 3 hinge points, the aortic annulus was seen in automatic multiplanar reconstruction. Annular measurements were obtained 0.5 mm below the hinge points, and the aortic valve annular contour was traced to calculate the perimeter-derived area and diameter (figure 1A). To define the direct one-planar measurement between the NCC and the LCC on the CCTA, a straight line between the red (LCC) and yellow (NCC) hinge points was used to determine length (figure 1B,C). The most appropriate THV size was selected based on size chart recommendations and anatomical considerations (figure 1D). CCTA-based measurements and calculations were used to determine the proper THV size according to commercial size charts supplied by the manufacturer. The mean diameter and area of the aortic annulus were used to select the THV size (23 mm, 26 mm, and 29 mm) and then coded as a binary variable for each size category; when only 1 measurement was within the proposed range for a specific THV size based on the manufacturer’s size chart (within the gray zone), the area was used to decide the final THV size.
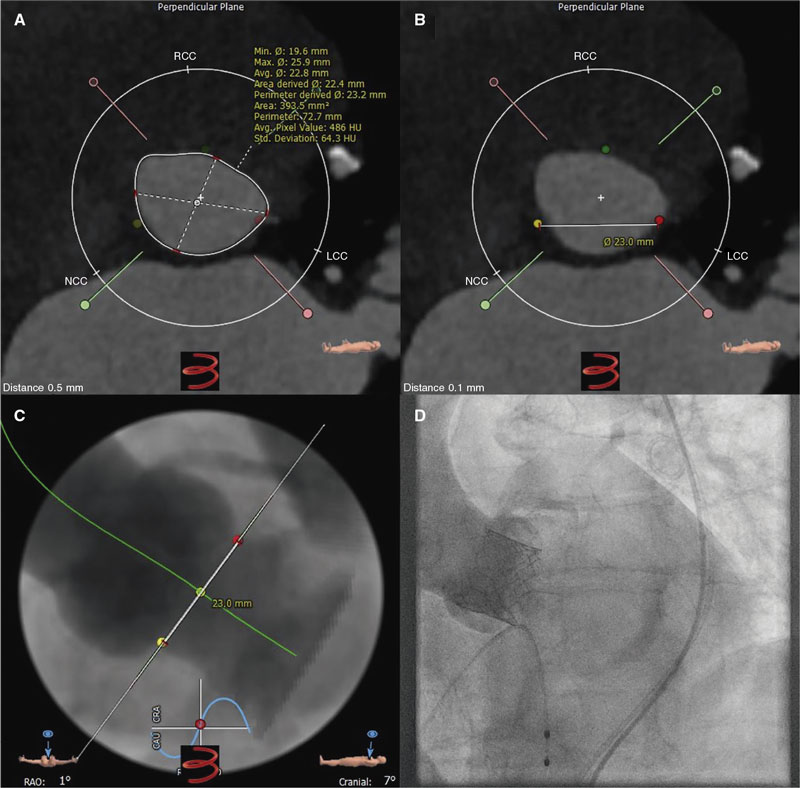
Figure 1. Central illustration. A: aortic annulus CCTA measurements determining the minimum (19.6 mm), maximum (25.9 mm), and mean (22.8 mm) diameter, perimeter (73 mm), and area (394 mm2). B: CCTA measurement from the lowest point between the left coronary cusp (red dot) and the non-coronary cusp (yellow dot) with a 23.0 mm distance. C: CCTA prediction of angiographic angulation to obtain the standard 3-cusp view (cranial right anterior oblique view of first and seventh nerves) with the left-coronary cusp from 1 side (red dot) and the non-coronary cusp on the other side (yellow dot), and the distance between them (23.0 mm). D: angiographic result after balloon-expandable valve implantation based on the tomographic measurements shown on figure 1 A (SAPIEN 3 Ultra 23 mm).
Aortographic measurements
All procedures were performed by experienced TAVI operators using a monoplane digital flat panel detector X-ray system (Allura Xper FD 10 C, Royal Philips, The Netherlands) in a dedicated hybrid cath lab. All fluoroscopic 3-cusp view images were analyzed after completion of the procedure and images with distance measurements saved. Angiographic measurements were obtained offline from the angiographic aortic root injection (native annulus without the implanted THV) using a 5-Fr pigtail catheter placed in the right coronary cusp in the standard 3-cusp view.14,15 The distance between the NCC and LCC hinge points was measured by experienced cardiologists (HA and JM) using dedicated Phillips software (figure 2A-H). Angiographic measurements were performed after automatic (based on calibration factor determined by the software) and manual (using the 5-Fr catheter as reference calibration factor) calibration to determine the distance in millimeters.
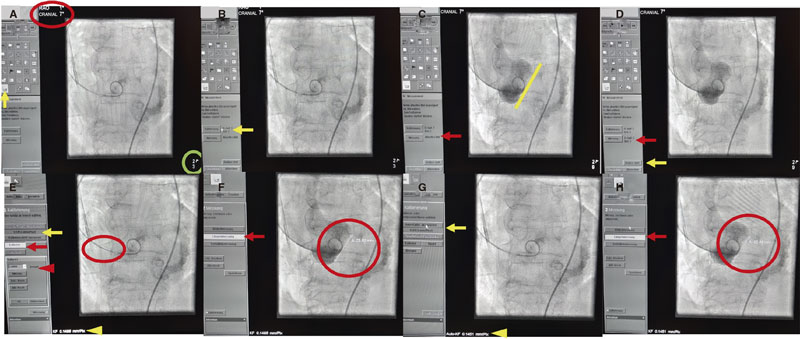
Figure 2. A: standard 3-cusp cranial right anterior oblique view of first and seventh nerves (red circle) to determine longitudinal measurement (yellow arow) in the best contrasted image (green circle). B: calibration option based on the best image available (yellow arrow). C: measurement option (red arrow) to determine the distance on the aortic annulus (yellow size) on the images saved (green circle). D: saved image (red arrow) to “start analysis” (yellow arrow). E: manual calibration based on the 5-Fr catheter (red arrow and arrowhead) drawing 2 lines over the catheter (red circle) comparing the calibration factor given by the software (0.1451 mm/pixels, yellow arrow) to the one obtained through manual calibration (0.1486 mm/pixels, yellow arrowhead). F: “longitudinal measurement” option should be selected (red arrow) drawing a line at the hinge point of the left and non-coronary cusp (red circle). G: for automatic calibration, select “accept automatic calibration” (yellow arrow); the calibration factor given by the software (0.1451 mm/pixels) will be used for measurement purpose (yellow arrowhead, calibration factor of 0.1451 mm/pixels). H: aortic valve annulus measurement using automatic calibration selecting “longitudinal measurement” (red arrow) and drawing a line between the hinge points of the left and non-coronary cusp (red circle).
Endpoints
Endpoints were the correlation between angiographic and CCTA measurements of the distance between the NCC and the LCC. The rates of the VARC-3-defined efficacy (technical success, correct position, and intended performance using VARC-319 definitions) and safety profile (multiple valves, valve embolization, pacemaker implantation, and more than moderate valvular regurgitation using VARC-3 definitions) in patients with concordant and discordant measurements between angiographic and CT-based measurements were also analyzed.
Rates of in-hospital complications defined as conversion to surgery, perioperative death, life-threating bleeding, major and minor bleeding, major and minor vascular complications, and in-hospital mortality in patients with concordant and discordant measurements were reported. The 30-day mortality rate, chronic heart failure, stroke, valve dysfunction, aortic mean gradient, and aortic regurgitation were reported too.
Statistical analysis
Categorical variables were expressed as frequencies and proportions and compared using the chi-square test or Fisher’s exact test, as appropriate. Continuous data were tested for normality with the Shapiro-Wilk test and expressed as mean (± standard deviation) or median (interquartile range [IQR]) as appropriate, and then compared, respectively, using the unpaired t test or the Mann-Whitney U test.
The study population was divided into derivation (n = 1256), and validation cohort (n = 40 cases). The study group of interest was obtained from the derivation cohort (n = 393). In the derivation cohort, selection of specific THV sizes (23 mm, 26 mm, and 29mm) based on the gold standard CCTA assessment was categorized as a binary variable and then compared to the THV size selection derived from aortography. Subsequently, logistic regression analysis was performed using the binary variable from the CCTA-based THV selection as a dependent variable while aortographic distance measurements were considered an independent variable. Afterwards, the receiver operating characteristic (ROC) curve was analyzed to identify optimal cut-off criteria (distance in mm, Youden’s index) and determine individual diameter ranges based on aortographic distance measurements of each category of THV sizes. The lowest value from the smallest THV and the highest value from the largest THV was determined using the 25th and 75th IQR, respectively, taken from the distribution of the derivation population. The suggested THV size was derived using aortography with manual or automatic calibration. Sensitivity, specificity, positive, and negative predictive values, as well as positive and negative likelihood were used to determine diagnostic accuracy index. Bland-Altman plots were used to test correlation between the CT NCC-LCC and the NCC-LCC aortography with manual calibration and NCC-LCC aortography with automatic calibration.
Inter- and intra-observer analysis using intraclass correlation coefficient (with absolute agreement) and Pearson correlation coefficient for dichotomized data were performed in a sample of 40 cases between the 2 independent cardiologists.
To perform internal validation, previously established cut-off values to determine THV size by aortography were applied in a separate cohort of 40 patients (validation cohort) and compared using the gold standard CCTA-based sizing determination.
Pairs of sizing results based on angiography and CCTA were generated as a study group of interest and classified as concordant or discordant after comparison using the chi square test or Fischer’s exact test, unpaired t test or the Mann-Whitney U test, as appropriate. Statistical analysis was performed using IBM SPSS Statistics software package (version 27, IBM Corporation, United States). All tests were 2-sided at the 0.05 significance level.
RESULTS
After exclusion, 1256/2500 (50.2%) patients who received a BEV were evaluated in the validation cohort (figure 3). Aortography-based diameter measurements were feasible in 393 of these patients (15.7%) (study group of interest).
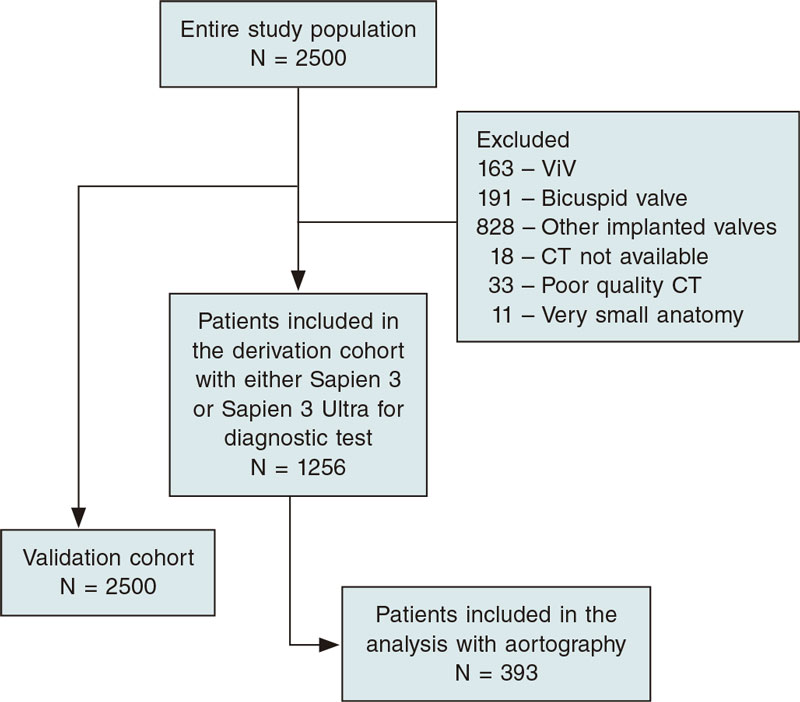
Figure 3. Study enrollment flow diagram.
Baseline and CCTA characteristics are shown on table 1. The median age of the entire population was 81 (77-85) years, 34.3% female, with a left ventricular function of 60% [47-60], and a median EuroScore II of 3.74 (2.14-6.24).
Table 1. Baseline characteristics
| Balloon-expandable valve (N = 393) | |
|---|---|
| Age, years | 81 [77 - 85] |
| Men | 257 (65.7) |
| BMI, kg/m2 | 26.2 [23.8-29.5] |
| BSA, m2 | 1.92 ± 0.22 |
| NYHA functional class III-IV | 238 (60.9) |
| CCS class III-IV | 32 (8.2) |
| Arterial hypertension | 353 (90.3) |
| Diabetes mellitus | 131 (33.5) |
| Dyslipidemia | 265 (67.8) |
| COPD | 19 (4.9) |
| Smoking history | 123 (31.5) |
| PAD | 77 (19.7) |
| Previous PCI | 162 (41.4) |
| CAD | 341 (87.2) |
| 1 vessel | 204 (52.2) |
| 2 vessels | 57 (14.6) |
| 3 vessels | 80 (20.5) |
| Pacemaker implantation | 36 (9.2) |
| Previous MI | 43 (11) |
| Previous CABG | 37 (9.5) |
| Previous Stroke/TIA | 60 (15.3) |
| Atrial fibrilation | 149 (38.1) |
| Creatinine levels, mg/dL | 1.11 [0.89-1.37] |
| eGRF, mL/min | 60 [46-76] |
| Dialysis | 3 (0.8) |
| Aortic regurgitation grade 2+ | 39 (10) |
| AVA, mm2 | 0.70 [0.60-0.84] |
| LVEF, % | 60 [47-60] |
| Mean Ao gradient, mmHg | 42 [36-49] |
| Peak Ao gradient, mmHg | 68 [59-80] |
| sPAP, mmHg | 42 [33-45] |
| EuroScore I, % | 11.84 [7.82-19.46] |
| EuroScore II, % | 3.74 [2.14 - 6.24] |
| CCTA measurements | |
| Minimum diameter, mm | 21.6 [20.1-23.2] |
| Maximum diameter, mm | 27.9 [26.2-29.6] |
| Mean diameter, mm | 24.8 [23.2-26.3] |
| Area, mm2 | 474 [414-533] |
| Perimeter, mm | 79 [74-84] |
| Visual estimate of the severity of valve calcification | |
| Mild | 80 (20.5) |
| Moderate | 185 (47.3) |
| Severe | 126 (32.2) |
| Visual estimate of the severity of annular calcification | |
| None | 55 (14.1) |
| Mild | 268 (68.5) |
| Moderate | 67 (17.1) |
| Severe | 1 (0.3) |
| Visual estimate of the severity of LVOT calcification | |
| None | 223 (57) |
| Mild | 145 (37.1) |
| Moderate | 23 (5.9) |
Data are expressed as no. (%), mean ± standard deviation or mean [interquartile range]. | |
Procedural characteristics are shown on table 2. Procedural time, fluoroscopic dose, and fluoroscopic time were 48 min [38-59], 919 [444-1712] cGys/cm2, and 10.9 min [8.2-14.7], respectively. Technical success was achieved in 95.4% of the cases. Regarding in-hospital complications (table 3), there rates of major bleeding events, major vascular complication, and in-hospital mortality were 16.1%, 14.6%, and 1.5%, respectively.
Table 2. Procedural characteristics
| Balloon-expandable valve (N = 393) | |
|---|---|
| Elective | 389 (99.5) |
| Need for intubation | |
| Prophylactic | 5 (1.3) |
| Emergency | 8 (2) |
| Use of ECMO | |
| Prophylactic | 0 (0) |
| Emergency | 1 (0.3) |
| Use of cerebral protection device | 16 (4.1) |
| Size of the valve implanted | |
| 23 mm | 118 (30.2) |
| 26 mm | 207 (52.9) |
| 29 mm | 66 (16.9) |
| THV implanted | |
| SAPIEN 3 | 95 (24.3) |
| SAPIEN 3 Ultra | 296 (75.7) |
| Predilatation | 166 (42.5) |
| Postdilatation | 54 (13.8) |
| Contrast media, mL | 139 [110-172] |
| Fluoroscopic time, min | 10.9 [8.2-14.7] |
| Fluoroscopic dose, cGys/cm2 | 919 [444-1712] |
| Procedural time, min | 48 [38 - 59] |
| Technical success | 373 (95.4) |
| Procedural success | 384 (98.2) |
| Intended performance | 380 (97.2) |
| Correct position | 389 (99.5) |
| Multiple valves | 1 (0.3) |
| Access site complications | 18 (4.6) |
| THV embolization | 1 (0.3) |
| Cardiac tamponade | 5 (1.3) |
| Annular rupture | 3 (0.8) |
| Coronary impairment | 0 (0) |
| Procedural CPR | 2 (0.5) |
| Conversion to surgery | 4 (1) |
| Procedural mortality | 3 (0.8) |
| Angiographic AR ≥ moderate | 5 (1.3) |
| Postoperative mean gradient, mmHg | 9 [5-10] |
| Days at the ICU | 1 [1-1] |
Data are expressed as no. (%), mean ± standard deviation or mean [interquartile range]. | |
Table 3. In-hospital complications
| Balloon-expandable valve (N = 393) | |
|---|---|
| Life-threatening bleeding | 11 (2.8) |
| Major bleeding | 63 (16.1) |
| Minor bleeding | 65 (16.6) |
| Major vascular complications | 57 (14.6) |
| Minor vascular complications | 80 (20.5) |
| TIA | 0 (0) |
| Major stroke | 4 (1) |
| Minor stroke | 5 (1.3) |
| Myocardial infarction | 3 (0.8) |
| New pacemaker implantation | 26 (6.6) |
| In-hospital mortality | 6 (1.5) |
Data are expressed as no. (%). | |
No differences were reported regarding the efficacy (94.2% vs 96%; P = .60) and safety profile (90.9% vs 91.9%; P = .84) between discordant and concordant pairs of tomographic and angiographic measurements using aortography with manual calibration, respectively (table 4).
Table 4. Procedural complications in concordant and discordant valve sizes using manual and automatic calibration in aortography vs CCTA (N = 393)
| All (N = 393) | Discordant (N = 121) | Concordant (N = 272) | P | |
|---|---|---|---|---|
| Efficacy | 375 (95.4) | 114 (94.2) | 261 (96.0) | .60a |
| Technical success | 386 (98.2) | 118 (97.5) | 268 (98.5) | .44b |
| Correct position | 391 (99.5) | 120 (99.2) | 271 (99.6) | .52b |
| Intended performance | 382 (97.2) | 117 (96.7) | 265 (97.4) | .74b |
| Safety | 360 (91.6) | 110 (90.9) | 250 (91.9) | .84a |
| Multiple valves | 1 (0.3) | 0 (0) | 1 (0.4) | > .99b |
| THV embolization | 1 (0.3) | 0 (0) | 1 (0.4) | > .99b |
| New pacemaker implantation | 27 (6.9) | 9 (7.4) | 18 (6.6) | .83a |
| AR > moderate after valve implantation | 5 (1.3) | 2 (1.7) | 3 (1.1) | .64b |
| Conversion to surgery | 4 (1.0) | 1 (0.8) | 3 (1.1) | > .99b |
| Procedural mortality | 3 (0.8) | 1 (0.8) | 2 (0.7) | > .99b |
| Life-threatening bleeding | 11 (2.8) | 5 (4.1) | 6 (2.2) | .32b |
| Major bleeding | 64 (16.3) | 20 (16.5) | 44 (16.2) | > .99a |
| Minor bleeding | 66 (16.8) | 16 (13.2) | 50 (18.4) | .24a |
| Major vascular complications | 58 (14.8) | 17 (14) | 41 (15.1) | .87a |
| Minor vascular complications | 81 (20.6) | 22 (18.2) | 59 (21.7) | .50a |
| In-hospital mortality | 6 (1.5) | 2 (1.7) | 4 (1.5) | > .99b |
Data are expressed as no. (%). | ||||
A moderate correlation was seen between CCTA-based assessment and aortographic THV size determination: 23 mm, 26 mm, and 29 mm THV sizes were associated with Spearman’s correlation coefficients of 0.528 (P < .01), 0.451 (P < .01), and 0.579 (P < .01), respectively. For more details, see tables 1-3 of the supplementary data.
The suggested angiographic cut-off values for each THV size are shown on table 5. In brief, the best diameter range for selecting 23 mm BEVs was 18.46 mm to 22.55 mm; for 26 mm THVs the best diameter range was 21.55 mm to 24.55 mm while for 29 mm THVs the best diameter range was ≥ 24.25 mm to < 28.50 mm. The intra- and inter-observer intraclass correlation coefficients were 0.931 (95%CI, 0.869-0.963; P < .01), and 0.902 (95%CI, 0.814-0.948; P < .01), respectively (see table 4 of the supplementary data). The CT NCC-LCC distance and NCC-LCC showed an intraclass correlation coefficient of 0.885 (95%CI, 0.834-0.920; P < .01) (figure 4).
Table 5. Suggested angiographic size chart for the Sapien balloon-expandable valve
| 23 mm | 26 mm | 29 mm | |
|---|---|---|---|
| N-L CC distance | 18.46–22.55 | 21.55–24.55 | 24.25–28.50 |
mm, millimeter; mm2, square millimeters; N-L CC, non-to-left coronary cusp. | |||
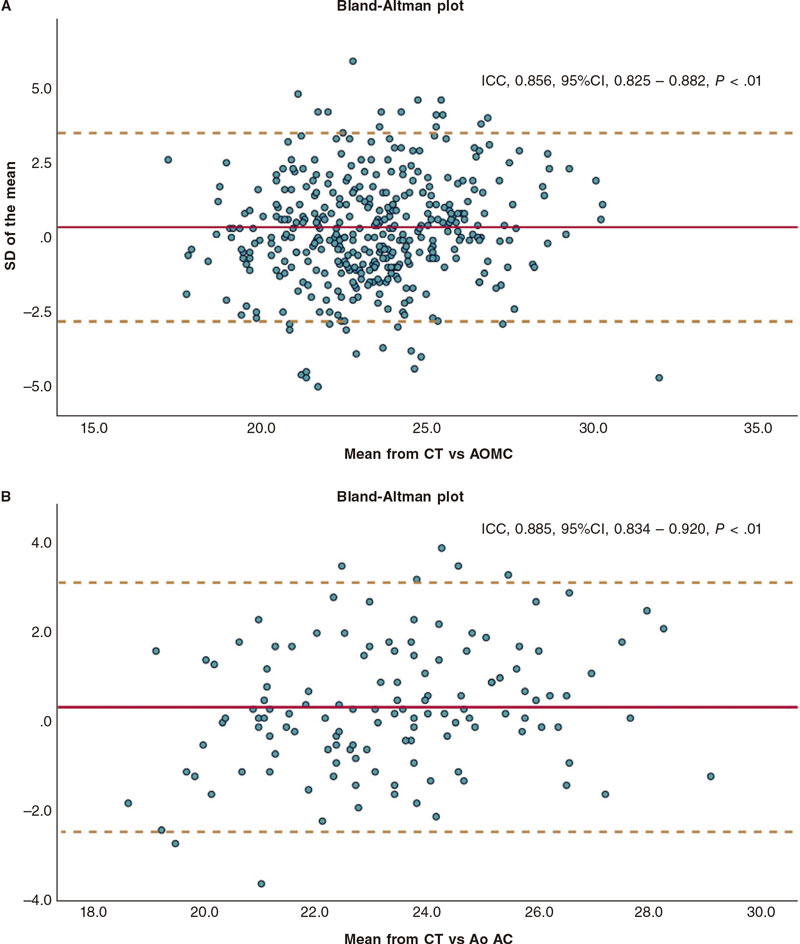
Figure 4. Bland-Altman plots: A: CCTA vs AOMC; B: CCTA vs AOAC. AOAC, aortography with automatic calibration; AOMC, aortography with manual calibration; CCTA, coronary computed tomography angiography; CI, confidence interval; ICC, intraclass correlation coefficient; SD, standard deviation.
The values obtained were tested and compared with the validation cohort (n = 40) showing moderate-to-good diagnostic test analysis with a good Spearman’s correlation coefficient [0.711 (95%CI, 0.506-0.840; P = < .01)], and moderate diagnostic accuracy (table 5 of the supplementary data). The validation cohort of 40 patients is shown on tables 6 to 10 of the supplementary data.
The 30-day follow-up is shown on table 6. There was no difference in 30-day mortality between discordant and concordant tomographic and angiographic measurements (1.7% vs 2.6%; P = .73). There was no difference at 30 days regarding the mean gradient (11 [10-16] vs 12 [10-15] mmHg; P = .76), and more than moderate aortic regurgitation (3.2% vs 1.1%; P = .34) using aortography with manual calibration between discordant and concordant tomographic and angiographic measurements, respectively.
Table 6. 30-day follow-up comparing concordant vs discordant measurements using balloon-expandable valve
| Total (N = 393) | Discordant (N = 121) | Concordant (N = 272) | P | |
|---|---|---|---|---|
| Mortality | 9 (2.3) | 2 (1.7) | 7 (2.6) | .72a |
| CHF | 24 (6.1) | 8 (6.6) | 16 (5.9) | .82b |
| Stroke | 2 (0.5) | 0 (0) | 2 (0.7) | > .99a |
| Valve dysfunction | 8 (2) | 2 (1.7) | 6 (2.2) | > .99a |
| LVEF, %, (n 278) | 60 [60-60] | 60 [57-60] | 60 [50-60] | < .01c |
| Mean gradient, mmHg (n = 265) | 12 [10-15] | 11 [10-16] | 12 [9.8-15] | .76c |
| AR > moderate, (n = 279) | 5 (1.8) | 3 (3.2) | 2 (1.1) | .33a |
| NYHA ≥ III, (n = 345) | 18 (5.2) | 5 (4.6) | 13 (5.5) | .80a |
Data are expressed as no. (%), mean ± standard deviation or mean [interquartile range]. | ||||
Discussion
This single-center, retrospective, observational study investigated the correlation and diagnostic accuracy between angiographic and tomographic measurements to determine THV size according to 1 single angiographic measurement between the NCC and the LCC in patients treated with BEV.
Regarding this objective, the most salient findings are a) angiographic aortic valve annular size determination based on distance measurements between the NCC and LCC is reproducible; b) diagnostic accuracy between CCTA-based and angiography-based aortic valve annular size determination is of moderate strength (table 5 of the supplementary data); and c) internal validation of previously established diameter ranges for angiography-based aortic valve annular size determination revealed moderate diagnostic accuracy.
We found a moderate overall diagnostic accuracy and correlation between angiographic and CCTA measurements to determine aortic valve annular size for THV selection. The use of angiography only measurements may expand the minimalistic TAVI approach in scenarios where CCTA is not an option or is unavailable.
The gold standard method to size the aortic annulus is direct surgical measurement, which is impossible in the TAVI setting. Hereby, several non-invasive reproducible methods have been used to determine the aortic valve annular size.3,5-7,20 However, the CCTA has been established as the gold standard diagnostic tool to determine aortic valve annular size dimensions4 due to its outstanding reproducibility. Before CCTA was established as the actual gold standard method, angiographic measurements were used demonstrating good correlation with direct perioperative measurements in patients undergoing surgical aortic valve replacement (r = 0.93).13 The comparison of transesophageal echocardiography and CCTA and direct perioperative measurements reported by Wang et al.20 showed a moderate correlation. Our study showed moderate diagnostic accuracy and correlation between angiographic and tomographic measurements to determine THV size. Similar results were previously tested in a small sample size of 50 patients where 60% of the valves were properly sized with fair-to-moderate agreement between angiography- and CCTA-guided selections.12 This provides evidence that angiography measurements could potentially be used in scenarios where CCTA is not available or its application is of increased risk.
Radiation exposure during CCTA assessment
It has been shown that TAVI-related imaging studies can potentially increase radiation exposure by some 15.4 to 79 mSv (millisieverts) with the TAVI procedure alone accounting for an effective dose of 26.9 ± 8 mSv and a dose-area product of 2006.3 ± 1152.2 cGys/cm2 (centiGrays/cm2). This radiation exposure is associated with a 70% and 50% increased risk of lung cancer-related death in women and men, respectively, and a 12% to 21%, and 23% to 33% risk of leukemia in women and men, respectively.10 We should mention that our study population experienced lower procedural radiation exposure (919 [444-1712] cGys/cm2) during TAVI including aortic valve annular sizing that may reduce radiation-associated risk of cancer. Using intraoperative low-dose radiation protocols can achieve equal efficacy in TAVI patients same as standard protocols without compromising safety,21 thus reducing radiation exposure and its associated risk. Additionally, the use of balloon-sizing combined with our proposed angiographic measurements may increase accuracy when determining the necessary THV size. Specifically, when the aortography annular measurement falls near the cut-off value between 2 different THV sizes, balloon-sizing can be used to confirm the use of the larger or smaller device.15 Although the additional use of balloon-sizing could increase radiation exposure due to additional imaging acquisition, the use of low-dose radiation protocol can reduce this risk without impacting the final result.21
Contrast media-associated nephropathy
Besides the benefits of mitigating radiation-associated risks, contrast media-associated nephropathy remains a critical concern in patients undergoing TAVI. Chronic kidney disease is present in around 38% of patients with aortic valve stenosis, 55%, 30%, and 15% of whom show mild, moderate, and severe chronic kidney disease.22 The use of contrast media can exacerbate acute kidney injury after its administration in patients with moderate-to-severe chronic kidney disease (from 2% to 17%)11 with a higher estimated 5-year mortality rate.22
Previous studies have demonstrated the safety and efficacy profile of TAVI compared to surgical aortic valve replacement across all ranges of surgical risk.23-28 Against this background, our data suggest that using aortography is safe to facilitate THV size selection in selected indications. In cases where aortography THV sizing was concordant with CCTA determined THV size, the safety and efficacy outcomes reported compared favorably to studies published in similar risk categories of patients.
Study limitations
This study is limited by its single-center observational nature. A randomized or prospective study may be needed to confirm our results. Furthermore, the use of 1 type of fluoroscopy equipment may limit the applicability of the findings to fluoroscopy equipment from alternative manufacturers. Additionally, due to data storage limitations, angiography was not always available to determine the aortic valve annular size through manual or automatic calibration. Upper and lower values of the suggested size chart were determined based on 75th and 25th interquartile range, respectively, due to the lack of upper or lower outliers that would allow us to determine these values.
CONCLUSIONS
Angiographic aortic valve annular measurements are reproducible and show moderate correlations and diagnostic accuracy compared to CCTA measurements when selecting the proper BEV THV size. This technique may be appropriate in situations when CCTA is not available, when high radiation exposure needs to be avoided, for patients in critical condition, and to reduce the risk of contrast-induced nephropathy. This strategy could potentially advance the minimalistic TAVI approach in selected patients.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
H. A. Alvarez-Covarrubias: conceptualization, methodology, formal analysis, investigation, resources, and original drafting of the manuscript. M. Kasel: conceptualization, original drafting of the manuscript, and editing. J.M. Michel: original drafting of the manuscript, and formal analysis. S. Cassese: visualization, investigation: S. Kufner: supervision, and data curation. C. Duesmann, C. Pellegrini, T. Rheude, and N. P. Mayr: resources, and data curation. H. Schunkert: supervision, drafting and revision of the manuscript, and visualization editing. A. Kastrati: supervision, visualization, drafting and revision of the manuscript, and editing. E. Xhepa: drafting of the manuscript, supervision, and formal analysis. G. Borrayo-Sánchez, and M. Joner: conceptualization, drafting and revision of the manuscript, and project administration.
CONFLICTS OF INTEREST
M. Kasel reports being a proctor and consultant for Edwards Lifesciences, but totally unrelated to this study; J. M. Michel reports a being proctor for Boston Scientific, but totally unrelated to this study; S. Cassese reports having received grants from Abbott Vascular, Boston Scientific, and SIS Medical AG, consulting fees from SIS Medical AG, and speaker fees from Abiomed, Astra Zeneca, SIS Medical AG, and Teleflex, but totally unrelated to this study; S. Kufner reports having received speaker and consultant fees from AstraZeneca, Bristol Myers Squibb, Bentley, and Translumina, but totally unrelated to this study; C. Pellegrini reports having received a grant from Else-Kröner Fresenius Memorial Stipendium, but totally unrelated to this study; T. Rheude reports having received lecturer fees from SIS Medical AG, and Astra Zeneca, but totally unrelated to this study; H. Schunkert reports having received consulting, honoraria, and speaker fees from AMGEN, Daiichi-Sankyo, MSD SHARP&DOHME, Astra Zeneca, Bayer Vital, Boehring-Ingelheim, Novartis, Servier, and Synlab, but totally unrelated to this study; A. Kastrati reports a patent number PCT/EP2021/053116, and participation on the Data Safety Monitoring Board of the DSMB-TARGET Trial, but totally unrelated to this study; E. Xhepa reports having received lecturer and speaker fees from Astra Zeneca, Boston Scientific, and SIS Medical AG, and support for attending meetings from Abbott Vascular, but totally unrelated to this study; G. Borrayo reports being former president of the Asociación Nacional de Cardiólogos de México from 2020 through 2022; M. Joner reports having received grants from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, Infraredx, consulting fees from Biotronik, TriCares, Veryan and Shockwave, and lecturer and speaker fees from Abbott Vascular, Biotronik, Boston Scientific, Edwards Lifesciences, Cardiac Dimensions, Astra Zeneca, Recor Medical, and Shockwave, but unrelated to this study; the remaining authors declared no conflicts of interest whatsoever. This manuscript is part of the Masters and PhD program in medical sciences of Universidad Nacional Autónoma de México (UNAM).
WHAT IS KNOWN ABOUT THE TOPIC?
- Little has been investigated in relation to the implementation of aortography as a diagnostic test to determine aortic valve annular size.
- Former studies used aortography to determine balloon size in valvuloplasty treatment in the pre-TAVI era.
- Aortography has been used in the TAVI era as a method to determine annular plane and for valve delivery purposes.
WHAT DOES THIS STUDY ADD?
- Implementation of aortography in addition to coronary computed tomography angiography (CCTA) may help us decide the size of the valve where gray zones areas are seen on the CCTA.
- Aortography measurements are reproducible and give moderate accuracy to decide the size of the valve in cases where CCTA is not available, and patients must be treated immediately.
REFERENCES
1. Clavel M-A, Messika-Zeitoun D, Pibarot P, et al. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. J Am Col Cardiol. 2013;62:2329-2338.
2. Kasel AM, Cassese S, Bleiziffer S, et al. Standardized Imaging for Aortic Annular Sizing: Implications for Transcatheter Valve Selection. JACC: Cardiovasc Imaging. 2013;6(2):249-62.
3. Francone M, Budde RPJ, Bremerich J, et al. CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol. 2020;30:2627-2650.
4. Blanke P, Weir-McCall JR, Achenbach S, et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC: Cardiovasc Imaging. 2019;12:1-24.
5. Fox H, Hemmann K, Lehmann R. Comparison of transthoracic and transesophageal echocardiography for transcatheter aortic valve replacement sizing in high-risk patients. J Echocardiogr. 2020;18:47-56.
6. Vo AT, Nakajima T, Nguyen TTT, et al. Aortic prosthetic size predictor in aortic valve replacement. J Cardiothorac Surg. 2021;16:221.
7. Cerillo AG, Mariani M, Berti S, Glauber M. Sizing the aortic annulus. Ann Cardiothorac Surg. 2012;1:245-256.
8. Okuno T, Heg D, Lanz J, et al. Heart valve sizing and clinical outcomes in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2021;98:E768-e79.
9. Medalion B, Blackstone EH, Lytle BW, White J, Arnold JH, Cosgrove DM. Aortic valve replacement: is valve size important? J Thorac Cardiovasc Surg. 2000;119:963-974.
10. Karambatsakidou A, Omar A, Chehrazi B, Rück A, Scherp Nilsson J, Fransson A. Skin dose, effective dose and related risk in transcatheter aortic valve implantation (TAVI) procedures: is the cancer risk acceptable for younger patients? Radiat Prot Dosimetry. 2016;169:225-231.
11. Davenport MS, Perazella MA, Yee J, et al. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294:660-668.
12. Gansera L, Ulm B, Bramlage P, et al. Utility of conventional aortic root shot angiography for SAPIEN 3 prosthesis sizing in TAVI: feasibility and inter-reader variability. Open Heart. 2019;6:e001201.
13. Mukharji J, Sloan TJ, Estrera AS, Lipscomb KM. Measurement of aortic root size by biplane angiography before cardiac valve replacement. Am J Cardiol. 1984;53:1084-1086.
14. Kasel AM, Cassese S, Leber AW, von Scheidt W, Kastrati A. Fluoroscopy-guided aortic root imaging for TAVR: “follow the right cusp” rule. JACC Cardiovasc Imaging. 2013;6:274-275.
15. Shivaraju A, Thilo C, Ott I, et al. Tools and Techniques - Clinical: Fluoroscopic balloon sizing of the aortic annulus before transcatheter aortic valve replacement (TAVR) - follow the “right cusp rule”. EuroIntervention. 2015;11:840-842.
16. Shivaraju A, Ott I, Cassese S, et al. Fluoroscopic calcification-guided optimal deployment projection during transcatheter aortic valve replacement - “The eye of the pigtail.” (Follow the right cusp rule- Part II). Catheter Cardiovasc Interv. 2016;87:996-998.
17. Kasel AM, Shivaraju A, Scheidt Wv, Kastrati A, Thilo C. Anatomic Guided Crossing of a Stenotic Aortic Valve Under Fluoroscopy: “Right Cusp Rule, Part III”. JACC: Cardiovasc Interv. 2015 Jan;8(1 Pt A):119-120.
18. Frangieh AH, Ott I, Michel J, et al. Standardized Minimalistic Transfemoral Transcatheter Aortic Valve Replacement (TAVR) Using the SAPIEN 3 Device: Stepwise Description, Feasibility, and Safety from a Large Consecutive Single-Center Single-Operator Cohort. Struct Heart. 2017;1:169-178.
19. Généreux P, Piazza N, Alu MC, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42:1825-1857.
20. Wang H, Hanna JM, Ganapathi A, et al. Comparison of aortic annulus size by transesophageal echocardiography and computed tomography angiography with direct surgical measurement. Am J Cardiol. 2015;115:1568-1573.
21. Michel JM, Hashorva D, Kretschmer A, et al. Evaluation of a Low-Dose Radiation Protocol During Transcatheter Aortic Valve Implantation. Am J Cardiol. 2021;139:71-78.
22. Bohbot Y, Candellier A, Diouf M, et al. Severe Aortic Stenosis and Chronic Kidney Disease: Outcomes and Impact of Aortic Valve Replacement. J Am Heart Assoc. 2020;9:e017190.
23. Leon MB, Smith CR, Mack M, et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N Engl J Med. 2010;363:1597-1607.
24. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N Engl J Med. 2011;364:2187-2198.
25. Thyregod HGH, Steinbrüchel DA, Ihlemann N, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis. J Am Coll Cardiol. 2015;65:2184-2194.
26. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321-1331.
27. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705.
28. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706-1715.
* Corresponding author.
E-mail address: gabyborsan@gmail.com (G. Borrayo-Sánchez).











