ABSTRACT
Introduction and objectives: Surgery for congenital heart defects with right ventricular outflow tract (RVOT) stenosis often results in significant pulmonary regurgitation, requiring pulmonary valve replacement in the long term. Despite the development of balloon-expandable prostheses, the native RVOT frequently dilates beyond the maximum diameters allowed for these valves. To allow percutaneous pulmonary valve implantation (PPVI) in these patients, clinical trials have been initiated with self-expanding prostheses, including the PULSTA valve. The aim of this study was to report the initial experience with this valve at three Spanish hospitals.
Methods: Descriptive study presenting the results of PPVI with the PULSTA prosthesis in patients with native RVOT and pulmonary regurgitation.
Results: We included 10 patients with a mean age of 15 ± 2.8 years. The implantation was successful in all patients, with no major complications occurring during the procedure. The mean length of follow-up was 18 [range, 2-35] months. In 8 patients, cardiac magnetic resonance was performed at 6 months, revealing a reduction in mean end-diastolic volume (131.7 ± 31.7 mL/m2 vs 100.3 ± 28.9 mL/m2) and end-systolic volume (68 ± 20.8 mL/m2 vs 57 ± 18.5 mL/m2).
Conclusions: The PULSTA prosthesis offers a safe, feasible, and effective alternative for PPVI in patients with native dilated RVOT. Due to the limited available follow-up data, further studies are needed to assess its long-term safety and durability.
Keywords: Congenital heart disease. Tetralogy of Fallot. Pulmonary regurgitation. Native right ventricular outflow tract. Transcatheter valve implantation. PULSTA valve.
RESUMEN
Introducción y objetivos: La cirugía de las cardiopatías congénitas con estenosis del tracto de salida del ventrículo derecho (TSVD) suele producir insuficiencia pulmonar con necesidad de recambio valvular a largo plazo. Pese al desarrollo de las prótesis expandibles con balón, los TSVD nativos corregidos con parche de ampliación pueden dilatarse por encima de los diámetros máximos admitidos para estas válvulas. Para posibilitar el implante percutáneo de válvula pulmonar (IPVP) en estos casos se están desarrollando prótesis autoexpandibles, entre las que se encuentra la PULSTA. El objetivo de este trabajo es presentar la experiencia inicial con esta válvula en 3 centros españoles.
Métodos: Estudio descriptivo de los resultados del IPVP con la prótesis PULSTA en pacientes con insuficiencia pulmonar sobre TSVD nativo.
Resultados: Se incluyeron 10 pacientes con una media de edad de 15 ± 2,8 años. En todos los casos se consiguió el implante sin complicaciones durante el procedimiento. El tiempo medio de seguimiento fue de 18 meses [rango 2-35 meses]. A 8 pacientes se les realizó una resonancia magnética cardiaca a los 6 meses, donde se observó una reducción de los volúmenes medios telediastólico (131,7 ± 31,7 frente a 100,3 ± 28,9 ml/m2) y telesistólico (68 ± 20,8 frente a 57 ± 18,5 ml/m2).
Conclusiones: La prótesis PULSTA ofrece una alternativa factible, segura y eficaz para el IPVP en pacientes con TSVD nativos dilatados. Son necesarios más estudios para evaluar su durabilidad y seguridad a largo plazo, ya que los datos de seguimiento son limitados.
Palabras clave: Cardiopatías congénitas. Tetralogía de Fallot. Insuficiencia pulmonar. Tracto de salida del ventrículo derecho nativo. Implante valvular percutáneo. Válvula PULSTA.
Abbreviations
CMR: cardiac magnetic resonance. LVEF: left ventricular ejection fraction. PPVI: percutaneous pulmonary valve implantation. RVOT: right ventricular outflow tract.
INTRODUCTION
Congenital heart diseases involving right ventricular outflow tract (RVOT) stenosis require surgical procedures that often compromise the function of the pulmonary valve. The main example of this is RVOT enlargement with transannular patch correction in Tetralogy of Fallot. Because of these patients’ current high survival rates (> 90% 25 years after surgical repair),1 they tend to develop hemodynamically significant pulmonary regurgitation, with an indication for valve replacement due to symptom onset or right ventricular dilatation or dysfunction, which is sometimes asymptomatic.2,3 To avoid the morbidity and mortality risk associated with repeat surgical procedures, percutaneous pulmonary valve implantation (PPVI) techniques have grown exponentially over the past 20 years, with excellent long-term results.4-6 These techniques have become the procedure of choice, and surgical aortic valve replacement is now reserved to anatomies ineligible for percutaneous approaches. In some patients with native RVOT, the volume overload due to pulmonary regurgitation leads to RVOT dilatation beyond the maximum diameters allowed for balloon-expandable valves—22 mm for the Melody TPV (Medtronic Inc., United States) and 29 mm for the Edwards SAPIEN XT THV and S3 (Edwards Lifescience, United States)—resulting, in recent years, in several clinical trials of self-expandable pulmonary valves with larger diameters to broaden the indications for PPVI to larger native RVOTs.7,8 The PULSTA valve (Taewoong Medical, South Korea) belongs to this new generation of self-expandable valves with promising initial results in small series in South Korea9,10 and Turkey.11 The objective of this study was to present the initial experience with this new valve in patients with dilatated native RVOT in 3 Spanish centers in Madrid, Spain.
METHODS
Patient selection
Hospitals La Paz and Gregorio Marañón are participating centers in the international multicenter clinical trial The PULSTA transcatheter pulmonary valve (TPV) pre-approval study (NCT03983512). The trial has just completed its enrollment phase and is currently analyzing the initial data. Of the 10 patients included in the present study, 8 are enrolled in this clinical trial, while the remaining 2 received the valve via compassionate use—1 at Hospital Universitario La Paz after the trial enrollment phase, and the other at Hospital Universitario 12 de Octubre.
We included participants with at least moderate pulmonary regurgitation after RVOT surgery due to initial obstructive lesions. The inclusion criteria were a) age ≥ 10 years and weight ≥ 30 kg; b) at least moderate pulmonary regurgitation in native RVOT with indications for valve replacement due to symptoms, worsening functional class, or progressive right ventricular dilatation or dysfunction on cardiac magnetic resonance (CMR); and c) pulmonary trunk measurements ≥ 16 mm and ≤ 30 mm as seen on the transthoracic echocardiogram, CMR, or computed tomography.
The degree of pulmonary regurgitation was assessed by both transthoracic echocardiography (table 1) and CMR, while considering the fraction of pulmonary regurgitation (< 20% mild, 20% to 40% moderate, and > 40% severe).
Table 1. Echocardiographic quantification criteria of pulmonary regurgitation
| Degree | Echocardiographic parameters |
|---|---|
| Mild | Narrow jet (≤ 1/3 of the pulmonary valve annulus diameter), weak continuous Doppler signal with slow deceleration |
| Moderate | Intermediate-sized jet (1/3 to 2/3 of the pulmonary valve annulus diameter), dense continuous Doppler signal |
| Severe | Wide jet (≥ 2/3 of the pulmonary valve annulus diameter), dense continuous Doppler signal with rapid flow deceleration or cessation in mid-to-late diastole, diastolic flow reversal in the pulmonary branches |
After the study was approved by the local ethics committees, the participants and their families were informed of the nature of the study, and gave their written informed consent to the indication and type of procedure. Ethical principles regarding privacy and confidentiality, as outlined in the Declaration of Helsinki of the World Medical Association revised in October 2013, were observed throughout the study.
The PULSTA Valve
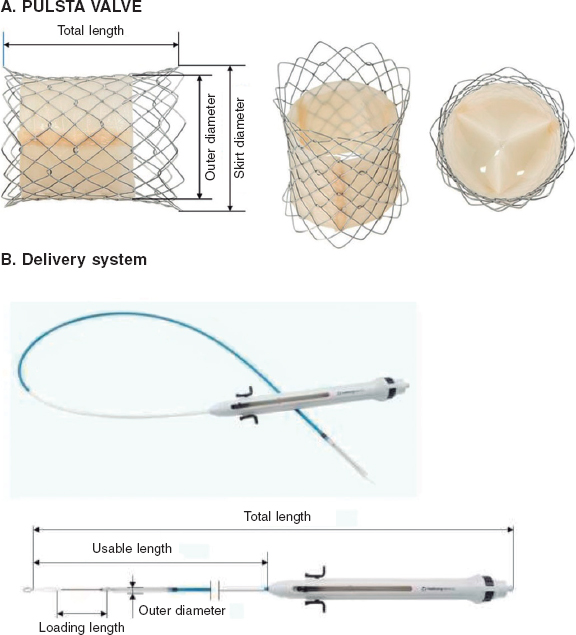
The PULSTA is a 3-leaflet porcine pericardial valve (decellularized and treated to prevent calcification) knitted to a self-expandable nitinol stent also covered with porcine pericardium, except for its proximal and distal portions, with radiopaque markers outlining the covered area (figure 1). The whole system has a diabolo-shaped configuration. The available diameters range from 18 mm to 32 mm—always in relation to the narrowest central area—with 2 mm increments. The diameter at the borders is always 4 mm larger, and total length varies between 28 mm and 38 mm and is diameter-dependent, with 1 row of uncovered proximal cells and 2 rows of distal cells to avoid obstructing flow through the pulmonary branches.
Figure 1. Image of the PULSTA valve showing its diabolo-shaped configuration and covering with its two ends uncovered (A) and the delivery system (B).
The length of the transport system (figure 1) is 110 mm, and its gauge for diameters of up to 28 mm and larger diameters is 18-Fr and 20-Fr, respectively. It requires simple pre-crimping at room temperature using a specific device, but no accessory sheaths to insert and navigate the system that would increase costs. The valve remains attached to the transport system through 3 small protrusions hooked on to the proximal cells. Once in position at the site of choice, the system is deployed by removing the covering portion, for which the proximal portion has a button that allows very fine movements and a trigger that releases the final portion. It reaches nominal diameters when nitinol reaches blood temperature. The valve is retrievable until the distal third is opened.
Valve implantation procedure
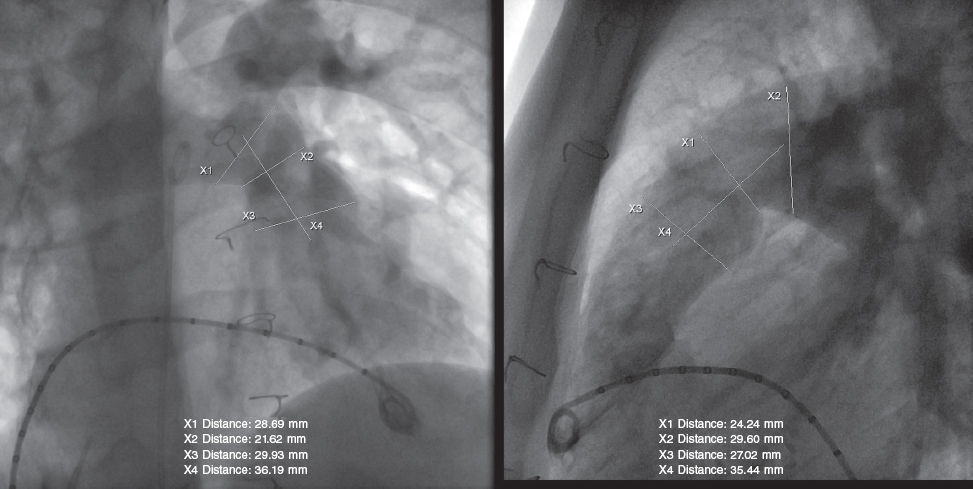
All procedures were performed under general anesthesia, while the patient remained on invasive mechanical ventilation and complete heparinization with a bolus of 100 IU/kg of sodium heparin. Two venous femoral vascular access es and 1 arterial access were cannulated. An initial hemodynamic study was conducted, with measurements of pressures on the right side and pulmonary angiography (figure 2) in several views (always with lateral and right anterior oblique views 30° more cranial), including sizing and examination of the pulmonary trunk dynamic behavior using a 34 mm AGA cutting balloon (AGA Medical Corporation, United States). A high-support guidewire (Lunderquist Cook, Denmark) was placed distal to the right or left branch, as appropriate, for navigation. The inflation of the measuring and sizing balloon was coordinated with selective coronary angiograms to rule out the risk of compression. Although the pre-CMR and quantitative angiography images supported the process, the angiographic measurement obtained with the cutting balloon had a more specific weight in the decision-making process. The manufacturer’s recommendation is to use valve sizes that should be 2 mm to 5 mm larger than the narrowest region of the pulmonary trunk. However, the final decision depends on the behavior and pulsatility of such region, the mean values of the entire length of the tract, the smaller diameter of the region, and the pre-bifurcation distal architecture of the branches. Each decision was made individually, considering other factors such as the presence of calcium in the RVOT enlargement patch, the patient’s weight, the caliber of the delivery system, and the proctor’s recommendations when available.
Figure 2. Pulmonary angiogram showing measurements during the pre-implantation assessment.
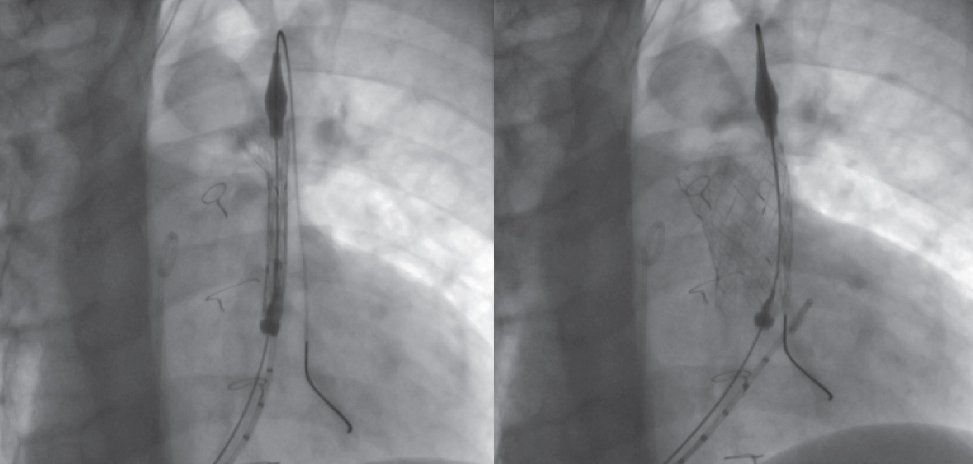
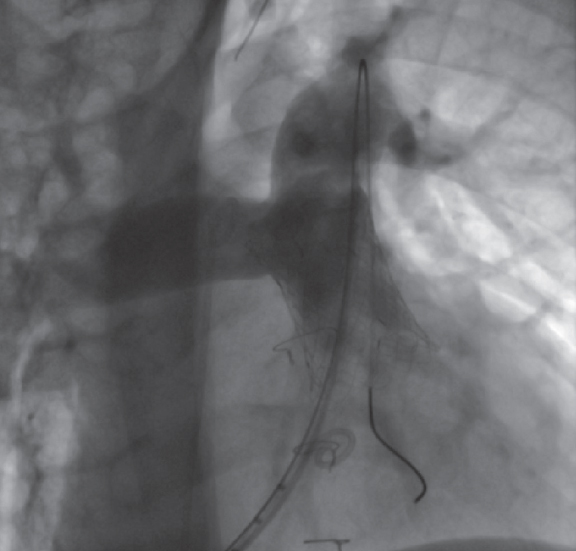
While mounted on the delivery system, the selected valve is moved toward the pulmonary trunk, its position is angiographically confirmed in the region of interest, and its cover is carefully removed (figure 3; videos 1 and 2 of the supplementary data). Correct positioning is facilitated by radiopaque markers. Once implanted, new measurements of right-sided pressures and a final pulmonary angiogram with a pigtail catheter in the same views are obtained to confirm the proper functioning of the valve (figure 4; video 3 of the supplementary data).
Figure 3. Sequence illustrating the gradual release of the valve inside the pulmonary trunk.
Figure 4. Final angiogram showing the valve implanted in the pulmonary trunk and absence of pulmonary regurgitation.
The procedure was considered successful when the device of the previously selected size was implanted, no acute complications occurred requiring removal such as coronary compression, or migration, and the final angiogram showed trivial or no pulmonary regurgitation.
The Perclose Pro-Glide system (Abbott, United States) was used to close the vascular access es through which the delivery system sheath was advanced. Simple compression was applied to all the remaining accesses.
Statistical analysis
In the descriptive analysis, continuous variables are expressed as mean ± standard deviation, or range, and categorical variables as frequencies and percentages. An exact permutation test was used to compare the variables at baseline and at 6 months after valve implantation. The analyses were performed using the STATA software package, version 17.0 (StataCorp-LLC, United States).
RESULTS
All 10 included patients met the inclusion criteria and received a PULSTA pulmonary valve in 1 of the 3 participating Spanish centers (Hospital Universitario La Paz, 6 patients; Hospital Universitario Gregorio Marañón, 3 patients; Hospital Universitario 12 de Octubre, 1 patient) from December 2019 through November 2022. Table 2 illustrates the participants’ baseline characteristics. The mean age and weight were 15 ± 2.8 (range, 13-23) years and 55.2 ± 19.5 (range, 30-87.8) kg, respectively. Eight of these patients (80%) were men. In most cases (80%), pulmonary regurgitation was secondary to transannular repair due to Tetralogy of Fallot, with 2 cases being due to pulmonary valve stenosis (1 associated with supravalvular pulmonary stenosis) that also required transannular RVOT enlargement. Two patients showed heart disease in a syndromic context: 1 had trisomy 21 and Tetralogy of Fallot, and the other had Noonan syndrome and pulmonary valve stenosis.
Table 2. Patients’ baseline characteristics
| Patient | Sex | Age (years) | Weight (kg) | Diagnosis | Valve indication | FC | Echo PR | CMR data | VO2peak (mL/kg/min) | % VO2theoretical peak | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RV EDV (mLm2) | RV ESV (mLm2) | RVEF (%) | PRF (%) | ||||||||||
| 1 | M | 16 | 59.3 | T. Fallot | PR + RV dilatation | I | Severe | 158.7 | 83.1 | 47.7 | 44.9 | 32.1 | 68 |
| 2 | M | 16 | 49.3 | T. Fallot | PR + clinical signs | II | Severe | 100.8 | 60.5 | 40 | 42.3 | 21.3 | 49 |
| 3 | M | 14 | 37.5 | T. Fallot | PR + RV dilatation | I | Severe | 166 | 97 | 41.7 | 70 | 38.9 | 80 |
| 4 | M | 13 | 30 | T. Fallot (Down Sd.) | PR + clinical signs | II | Severe | 126.6 | 60.7 | 52.03 | 40.9 | - | - |
| 5 | M | 14 | 56.4 | Valvular and supravalvular PS | PR + clinical signs | II | Severe | 83.3 | 36.8 | 55.8 | 35.6 | 39.8 | 69 |
| 6 | W | 14 | 87.8 | T. Fallot | PR + clinical signs | II | Severe | 108 | 43 | 60 | 36 | 28.4 | 100 |
| 7 | W | 15 | 47 | Valvular PS (Noonan Sd.) | PR + RV dilatation | I | Severe | 115 | - | 53 | 47 | - | - |
| 8 | M | 13 | 35 | T. Fallot | PR + RV dilatation | I | Severe | 165.7 | 93.9 | 43 | 47 | - | - |
| 9 | M | 15 | 67.5 | T. Fallot | PR + RV dilatation | I | Severe | 120 | 69.8 | 42 | 51 | - | - |
| 10 | M | 23 | 82 | T. Fallot | PR + RV dilatation | I | Severe | 173 | 67 | 55 | 45 | - | - |
|
CMR, cardiac magnetic resonance; echo PR, echocardiographic assessment of pulmonary regurgitation; EDV, end-diastolic volume; FC, functional class; M, man; PR, pulmonary regurgitation; PRF, pulmonary regurgitation fraction; PS, pulmonary stenosis; RV, right ventricle; RVEF, right ventricular ejection fraction; Sd, syndrome; T, tetralogy; VO2: oxygen volume; W, woman. |
|||||||||||||
According to the CMR, the mean right ventricular volumes were 131.7 ± 31.7 mL/m2 (end-diastolic) and 68 ± 20.8 mL/m2 (end-systolic), and the mean right ventricular ejection fraction (RVEF) was 49% (range, 40% to 60%). The mean pulmonary regurgitant fraction was 46% (range, 35.6% to 70%). The mean maximal oxygen consumption was 32.1 ± 7.7 mL/kg/min, and 4 of the 5 patients who underwent ergospirometry showed oxygen consumption < 80% of the expected level for their age and weight.
Table 3 illustrates the hemodynamic and angiographic measurements and procedural data. None of the patients had significant residual RVOT stenosis, although an AndraStent 30 XL stent (Andramed, Germany) had been previously implanted in the left pulmonary artery in 1 patient due to stenosis. The mean RVOT-pulmonary artery pressure gradient was 7.2 ± 4.7 mmHg. Valve size was 26 mm in 1 patient, 28 mm in 2 patients, 30 mm in 6 patients, and 32 mm in 1 patient. In 1 patient, the valve was placed inside a stent previously implanted in the RVOT (CP 10 ZIG 50 mm stent, NuMED, United States) and was dilated with a high-pressure balloon up to 30 mm. In all patients, implantation was performed via femoral vascular access. The mean procedural and fluoroscopy times were 165 (range, 122 to 233) minutes and 30 (range, 18 to 50) minutes. All valves were successfully implanted with no acute complications during the procedure. The pulmonary regurgitation seen on the final angiogram was trivial or nonexistent.
Table 3. Hemodynamic and angiographic measurements and procedural data
| Patient | Initial angio PR | Initial PG RV-PA (mmHg) | Previous stents | PT measurement (mm) | Measurement cutting balloon (mm) | PULSTA valve size (mm) | Proc t (min) | Fluoro t (min) | Final angio PR | Final PG RV-PA (mmHg) | Post echo PR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Severe | 14 | No | 26.6 | 26 | 30 | 145 | 22 | No | 0 | No |
| 2 | Severe | 0 | AndraStent 30 XL in RPB | 23 | 28 | 28 | 135 | 18 | No | 6 | No |
| 3 | Severe | 3 | No | 30 | 25 | 30 | 140 | 28 | Trivial | 2 | Mild |
| 4 | Severe | 12 | No | 30.1 | 27.5 | 28 | 195 | 40 | Trivial | 7 | No |
| 5 | Severe | 9 | No | 27.6 | 24 | 30 | 170 | 24 | No | 6 | No |
| 6 | Severe | 8 | CP 10 ZIG 50 mm in PT | 26 | 28 | 30 | 160 | 29 | No | 6 | No |
| 7 | Severe | 7 | No | 31 | 24.5 | 30 | 159 | 50 | No | 2 | No |
| 8 | Severe | 10 | No | 26 | 18.6 | 26 | 122 | 26 | No | 4 | No |
| 9 | Severe | 2 | No | 27 | 25 | 32 | 233 | 37 | No | 3 | No |
| 10 | Severe | 0 | No | 29 | 30 | 30 | 195 | 28 | Trivial | 5 | Mild |
|
angio PR, angiographic assessment of pulmonary regurgitation; fluoro t, fluoroscopy time; PG RV-PA, peak pressure gradient right ventricle-pulmonary artery (invasive measurement); post echo PR, pulmonary regurgitation in the first ECG after the procedure; proc T, procedural time; PT, pulmonary trunk. |
|||||||||||
Four patients (40%) experienced adverse events after implantation: 2 developed chest pain the evening following the procedure. In both patients, an ECG was performed showing no changes compared with the baseline values (both showed repolarization changes in precordial leads due to pre-existing right bundle branch block), preserved biventricular function without segmental contractility alterations and no pericardial effusion. Thoracic computed tomography ruled out the presence of coronary compression, and the blood test results showed no elevation of troponin levels. Pain subsided with standard analgesia (IV metamizole), and the patients remained asymptomatic. A third patient developed monomorphic ventricular extrasystoles without clinical repercussions a few hours after the procedure, and treatment with atenolol was initiated, resulting in good control. Treatment was discontinued at 6 months, with no recurrence of extrasystoles. The fourth patient experienced self-limiting mild hemoptysis that required no treatment. All patients were discharged 24 to 72 hours after the procedure on antiplatelet doses of aspirin.
Follow-up data after implantation are shown in table 4. The median length of follow-up after the procedure was 18 (range, 2-35) months. Eight out of the 10 participants underwent a follow-up CMR at 6 months that showed reduced mean end-diastolic (131.7 ± 31.7 mL/m2 before vs 100.3 ± 28.9 mL/m2 at 6 months) and end-systolic volumes (68 ± 20.8 mL/m2 before vs 57 ± 18.5 mL/m2 at 6 months). However, this reduction was not statistically significant (P = .065 and P = .49, respectively).
| Patient | Foll t (months) | RV EDV (mL/m2) | RV ESV (mL/m2) | FC | Last echo PR | Last echo PF RV-PA (mmHg) | Complications | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |||||
| 1 | 26 | 158.7 | 102 | 83.1 | 57.9 | I | I | No | 12 | Self-limiting chest pain |
| 2 | 35 | 100.8 | 74.7 | 60.5 | 41.2 | II | I | No | 15 | No |
| 3 | 33 | 166 | 125.8 | 97 | 78.7 | I | I | Moderate | 15 | Self-limiting chest pain |
| 4 | 25 | 126.6 | 80 | 37 | 37 | II | I | Mild | 11 | No |
| 5 | 12 | 83.3 | 70.5 | 36.8 | 37.9 | II | I | No | 11 | No |
| 6 | 3 | 108 | 43 | II | II | No | 13 | No | ||
| 7 | 25 | 115 | 81 | I | I | No | 14 | Self-limiting mild hemoptysis | ||
| 8 | 6 | 165.7 | 151.1 | 93.9 | 74.5 | I | I | Mild | 14 | Ventricular extrasystole treated with atenolol |
| 9 | 9 | 120 | 117.2 | 69.8 | 73.3 | I | I | No | 15 | No |
| 10 | 2 | 173 | 67 | I | I | Mild | - | No | ||
|
EDV, end-diastolic volume; ESV, end-systolic volume; FC, functional class; foll t, follow-up time; PG RV-PA, peak pressure gradient right ventricle-pulmonary artery; PR, pulmonary regurgitation; RV, right ventricle. |
||||||||||
The 6-month follow-up ECG revealed moderate intraprosthetic pulmonary regurgitation suggestive of valve dysfunction in 1 patient. This finding was later confirmed by a CMR showing a 32.7% regurgitant fraction (compared with the 70% found prior to valve implantation). Since the patient remained asymptomatic and right ventricular volumes had reduced, a wait-and-see approach was adopted. In this patient, pulmonary regurgitation remained moderate 33 months after implantation. Among the remaining patients, 6 showed no pulmonary regurgitation and 3 showed mild regurgitationin the last follow-up ECG. Three out of the 4 patients with exercise deterioration improved to functional class I, and 1 remained in functional class II.
None of the patients died during follow-up, and there were no serious device malfunctions requiring replacement. Although stent fractures were unlikely due to the relatively short follow-up and design of the valve that used a nitinol mesh with interlacing cells rather than welding, making it more resistant to this complication, chest x-rays were obtained from 8 patients 6 months after implantation. No abnormalities were found. No cases of infective endocarditis or ventricular arrhythmias were reported beyond the immediate postoperative period.
DISCUSSION
In our series of patients with a history of right sided obstructive congenital heart disease and pulmonary regurgitation in the native RVOT, the initial results with the PULSTA valve are promising. The implantation success rate was 100%, there were no serious acute complications, and right ventricular volumes decreased on CMR 6 months after the procedure.
Two patients experienced nonspecific chest pain a few hours after implantation, with no signs of coronary compression, ECG changes or elevated troponin levels. In both patients, the chest pain was resolved with standard analgesia This symptom has already been reported in previous series,9-11 and is attributed to device-induced distension of the pulmonary arterial wall.
One patient developed moderate intraprosthetic pulmonary regurgitation 6 months after implantation but has remained stable ever since without symptoms or right ventricular dilatation on imaging modalities. No previous series have reported the development of significant pulmonary regurgitation during follow-up,9-11 highlighting the need for further studies with a larger number of patients and longer follow-up periods to assess the durability of the PULSTA valve.
Pulmonary regurgitation is a common residual lesion in patients undergoing surgery for right heart obstructive lesions, with long-term effects on right ventricular function and exercise capacity.12 Although balloon-expandable pulmonary valves have yielded good international results, patients with large native RVOTs have historically been excluded from PPVI.13 To address this limitation, several large-diameter self-expandable pulmonary valves, including the PULSTA, are currently in the pipeline.
The Venus P-Valve (Venus Medtech, China) is another self-expandable pulmonary valve designed for native RVOTs and already has the CE marking. Initial series have reported high implantation success rates and good short- and mid-term results,14-16 similar to the PULSTA valve. The advantage of both devices is that they can be implanted in a single procedure after the initial diagnostic assessment because they do not require previous stent implantation to create a scaffold for valve implantation, as is the case with balloon-expandable valves. In our opinion, the PULSTA valve is particularly suitable for the pediatric population due to its slightly smaller profile (maximum length, 38 mm), which facilitates navigation and implantation in the curved anatomy of the RVOT, and smaller delivery system (18-Fr or 20-Fr compared with the 22-Fr or 24-Fr of the Venus P-Valve), which reduces the risk of vascular injury in smaller patients. However, there are currently no studies that compare the 2 self-expandable valves.
Limitations
The main limitations of this study are its small sample size and relatively short follow-up, limiting its statistical power and ability to detect rare adverse events, or long-term occurrences. Possible sex and gender variables in accordance with the SAGER guidelines have also not been taken into account.
CONCLUSIONS
Based on our initial experience, the PULSTA valve is a feasible, safe, and effective alternative to PPVI in most patients with dilated native RVOTs who would have otherwise required surgery. However, more studies are needed to evaluate its long-term durability and safety profile since the current follow-up data are still limited.
FUNDING
Eight out of the 10 study participants are enrolled in the PULSTA transcatheter pulmonary valve pre-approval study (registration no. NCT03983512) funded by Taewoong Medical (South Korea).
ETHICAL CONSIDERATIONS
The Ethics Committees of La Paz and Gregorio Marañón Hospitals approved the inclusion of patients in the study. Informed consents were obtained from the patients after receiving information adapted to their age, and from the parents in those cases under 18 years of age.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence was not used for the development of this study or writing of the manuscript.
AUTHORS’ CONTRIBUTIONS
All the authors participated in the treatment and follow-up of the included patients. D. Salas-Mera, A. Sobrino, and F. Sarnago collected data from each participating center. D. Salas-Mera, E. Balbacid, C. Abelleira, and F. Gutiérrez-Larraya analyzed the data and drafted the manuscript. All authors participated in the data interpretation, critical review process, and final approval of the manuscript.
CONFLICTS OF INTEREST
D. Salas-Mera, C. Abelleira, E. Balbacid, A. Sobrino, J.L. Zunzunegui, and F. Gutiérrez-Larraya are participating investigators in the international and multicenter PULSTA transcatheter pulmonary valve pre-approval study. The remaining authors declare no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- Pulmonary regurgitation is a common residual lesion after the surgical repair of congenital heart diseases involving obstructive lesions of the right ventricular outflow tract. Despite successful international experiences with balloon-expandable valves for percutaneous pulmonary valve replacement, the native outflow tract often dilates beyond the maximum diameters allowed by these valves. To enable percutaneous valve implantation in these cases, a new generation of self-expanding valves is currently in the pipeline.
WHAT DOES THIS STUDY ADD?
- We report the first series of patients who received the PULSTA self-expanding pulmonary valve in Spain. The good initial results in terms of safety and efficacy make it an attractive option for percutaneous pulmonary valve implantation in patients with pulmonary regurgitation and dilated native right ventricular outflow tracts.
SUPPLEMENTARY DATA
Video 1. Salas-Mera D. DOI: 10.24875/RECICE.M23000402
Video 2. Salas-Mera D. DOI: 10.24875/RECICE.M23000402
Video 3. Salas-Mera D. DOI: 10.24875/RECICE.M23000402
REFERENCES
1. Smith CA, McCracken C, Thomas AS, et al. Long-term Outcomes of Tetralogy of Fallot:A Study From the Pediatric Cardiac Care Consortium. JAMA Cardiol. 2019;4:34-41.
2. Baumgartner H, De Backer J, Babu-Narayan SV, et al.;ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563-645.
3. Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease:A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698-e800.
4. Cools B, Brown S, Budts W, et al. Up to 11 years of experience with the Melody valved stent in the right ventricular outflow tract. EuroIntervention. 2018;14:e988-e994.
5. McElhinney DB, Zhang Y, Levi DS, et al. Reintervention and Survival After Transcatheter Pulmonary Valve Replacement. J Am Coll Cardiol. 2022;79:18-32.
6. Lawley CM, Tanous D, O'Donnell C, et al. Ten Years of Percutaneous Pulmonary Valve Implantation in Australia and New Zealand. Heart Lung Circ. 2022;31:1649-1657.
7. Morgan G, Prachasilchai P, Promphan W, et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve:international experience. EuroIntervention. 2019;14:1363-1370.
8. Giugno L, Faccini A, Carminati M. Percutaneous Pulmonary Valve Implantation. Korean Circ J. 2020;50:302-316.
9. Kim AY, Jung JW, Jung SY, et al. Early Outcomes of Percutaneous Pulmonary Valve Implantation with Pulsta and Melody Valves:The First Report from Korea. J Clin Med. 2020;9:2769.
10. Lee SY, Kim GB, Kim SH, et al. Mid-term outcomes of the Pulsta transcatheter pulmonary valve for the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2021;98:E724-E732.
11. Odemis E, Yenidogan I, Kizilkaya MH. Early results of PULSTA transcatheter heart valve in patients with enlarged right ventricular outflow tract and severe pulmonary regurgitation due to transannular patch. Cardiol Young. 2022;16:1-9.
12. Bouzas B, Kilner PJ, Gatzoulis MA. Pulmonary regurgitation:not a benign lesion. Eur Heart J. 2005;26:433-439.
13. Ansari MM, Cardoso R, Garcia D, et al. Percutaneous Pulmonary Valve Implantation:Present Status and Evolving Future. J Am Coll Cardiol. 2015;66:2246-2255.
14. Sivakumar K, Sagar P, Qureshi S, et al. Outcomes of Venus P-valve for dysfunctional right ventricular outflow tracts from Indian Venus P-valve database. Ann Pediatr Cardiol. 2021;14:281-292.
15. Garay F, Pan X, Zhang YJ, Wang C, Springmuller D. Early experience with the Venus p? valve for percutaneous pulmonary valve implantation in native outflow tract. Neth Heart J. 2017;25:76-81.
16. Morgan G, Prachasilchai P, Promphan W, et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve:international experience. EuroIntervention. 2019;14:1363-1370.