ABSTRACT
Introduction and objectives: A systematic approach to patients with angina with no obstructed coronary arteries (ANOCA) or ischemia with no obstructed coronary arteries (INOCA) patients is not routinely implemented.
Methods: All consecutive patients diagnosed with ANOCA/INOCA were referred to a designated outpatient clinic for a screening visit to assess their eligibility for a NOCA program. If eligible, patients underwent scheduled coronary angiograms with coronary function testing and intracoronary acetylcholine provocation testing. Medical therapy was optimized accordingly. All patients were then followed up at 1, 3, 6, and 12 months. Baseline and 3-month follow-up assessments included the Seattle Angina Questionnaire (SAQ) and EuroQol-5D questionnaire.
Results: Of 77 patients screened, 23 (29.9%) were excluded and 54 (70.1%) were included (29 [53.7%] with INOCA and 25 [46.3%] with ANOCA). Microvascular angina was diagnosed in 19 (35.2%) patients, vasospastic angina in 12 (22.2%), both microvascular angina and vasospastic angina in 18 (33.3%), and noncoronary chest pain in 5 (9.3%). There was a notable increase in the use of beta-blockers, calcium channel blockers and nitrates. Complications occurred in 3 (5.5%) patients. Compared with baseline, there was no difference in the mean EQ-5D score at the 3-month follow-up, but there was a significant improvement in the SAQ score related to physical limitations, angina stability, and disease perception, with no differences in angina frequency or treatment satisfaction. No events were recorded at the 1-year follow-up.
Conclusions: A specific diagnostic and therapeutic protocol can be easily and safely implemented in routine clinical practice, leading to improvement in patients’ quality of life.
Keywords: INOCA. ANOCA. Diagnosis. Therapy. Protocol.
RESUMEN
Introducción y objetivos: El abordaje sistemático en pacientes con angina con arterias coronarias no obstruidas (ANOCA) o con isquemia con arterias coronarias no obstruidas (INOCA) no está bien protocolizado.
Métodos: Todos los pacientes con diagnóstico de INOCA o ANOCA se trasladaron a una clínica ambulatoria específica para evaluar su elegibilidad para el programa NOCA. Si eran elegibles, se sometían a una angiografía coronaria programada con pruebas de función coronaria y provocación intracoronaria con acetilcolina. La terapia médica se optimizó en consecuencia. Todos los pacientes tuvieron un seguimiento a 1, 3, 6 y 12 meses. Al inicio y a los 3 meses se aplicaron los cuestionarios SAQ y EuroQol-5D.
Resultados: De 77 pacientes se excluyeron 23 (29,9%) y se incluyeron 54 (70,1%) (29 [53,7%] con INOCA y 25 [46,3%] con ANOCA). Se diagnosticó angina microvascular a 19 (35,2 %) pacientes, angina vasoespástica a 12 (22,2 %), angina microvascular y angina vasoespástica a 18 (33,3 %), y dolor torácico no coronario a 5 (9,3 %). Hubo un aumento significativo en el uso de bloqueadores beta, bloqueadores del calcio y nitratos. Se presentaron complicaciones en 3 (5,5%) pacientes. No hubo diferencias en la puntuación media del EQ-5D a los 3 meses y se observó una mejora significativa en la puntuación SAQ respecto a la limitación física, la estabilidad de la angina y la percepción de enfermedad, sin diferencias en la frecuencia de angina y la satisfacción con el tratamiento. No se registraron eventos al año.
Conclusiones: Un protocolo diagnóstico y terapéutico específico podría implementarse de manera fácil y segura en la práctica clínica diaria, y con ello mejoraría la calidad de vida de los pacientes.
Palabras clave: INOCA. ANOCA. Diagnóstico. Terapia. Protocolo.
Abbreviations ANOCA: angina with no obstructed coronary arteries. INOCA: ischemia with no obstructed coronary arteries.
INTRODUCTION
Ischemic heart disease is the leading cause of disability and mortality worldwide and is commonly characterized by the presence of obstructive coronary artery disease (CAD) (defined as any coronary artery stenosis ≥ 50% in diameter).1 However, up to 60% to 70% of patients with angina and/or documented myocardial ischemia do not have angiographic evidence of CAD.2 This condition is defined as angina with no obstructed coronary arteries (ANOCA) or ischemia with no obstructed coronary arteries (INOCA) when associated with evidence of myocardial ischemia.3 Of note, despite the absence of CAD, these patients are at an increased risk of future cardiovascular events such as acute coronary syndrome, heart failure hospitalization, stroke, and repeat cardiovascular procedures compared with healthy individuals.4,5 Therefore, appropriate management in terms of diagnosis and treatment is of the utmost importance to improve patients’ prognosis and outcomes.6 The Coronary Microvascular Angina (CorMicA) trial demonstrated that a strategy of adjunctive invasive testing for disorders of coronary function together with stratified medical therapy can improve outcomes (ie, reduction in angina severity and enhanced quality of life).7,8 However, there are still concerns about the implementation in real-world practice of a systematic diagnostic and therapeutic approach in INOCA and ANOCA patients, potentially impacting outcomes and quality of life.
We report our single-center experience of the implementation in clinical practice of a specific diagnostic and therapeutic protocol (no obstructed coronary arteries [NOCA] program) in INOCA and ANOCA patients.
METHODS
Eligibility criteria for the NOCA program
All consecutive patients diagnosed either at our hospital or at our referral centers with angina or ischemia with nonobstructive CAD on coronary angiography were referred to a specific outpatient clinic (the NOCA clinic at Hospital Clínic, Barcelona, Spain) for a screening visit. Nonobstructive CAD was defined as angiographic evidence of normal coronary arteries or diffuse atherosclerosis with stenosis < 50% and/or fractional flow reserve (FFR) > 0.80 if there was stenosis between 50% and 70%. During the screening visit, a team of expert cardiologists confirmed patients’ eligibility for the NOCA program based on the following criteria: a) diagnosis of ANOCA, defined as stable, chronic typical angina symptoms (eg, chest pain precipitated by physical exertion or emotional stress and relieved by rest or nitroglycerine); b) diagnosis of INOCA, defined as the demonstration of myocardial ischemia identified by a noninvasive test with pharmacologic or exercise stress tests such as cardiac single photon emission computed tomography, cardiac magnetic resonance, stress electrocardiography, or echocardiography.3 The exclusion criteria were: a) atypical angina symptoms, and b) clearly identifiable noncoronary causes of chest pain (figure 1). The study protocol adhered to the Declaration of Helsinki and the study was approved by our institutional review committee. All patients provided written informed consent to be included in this program and study. The clinical ethics committee gave their approval for a retrospective analysis of the collected data.
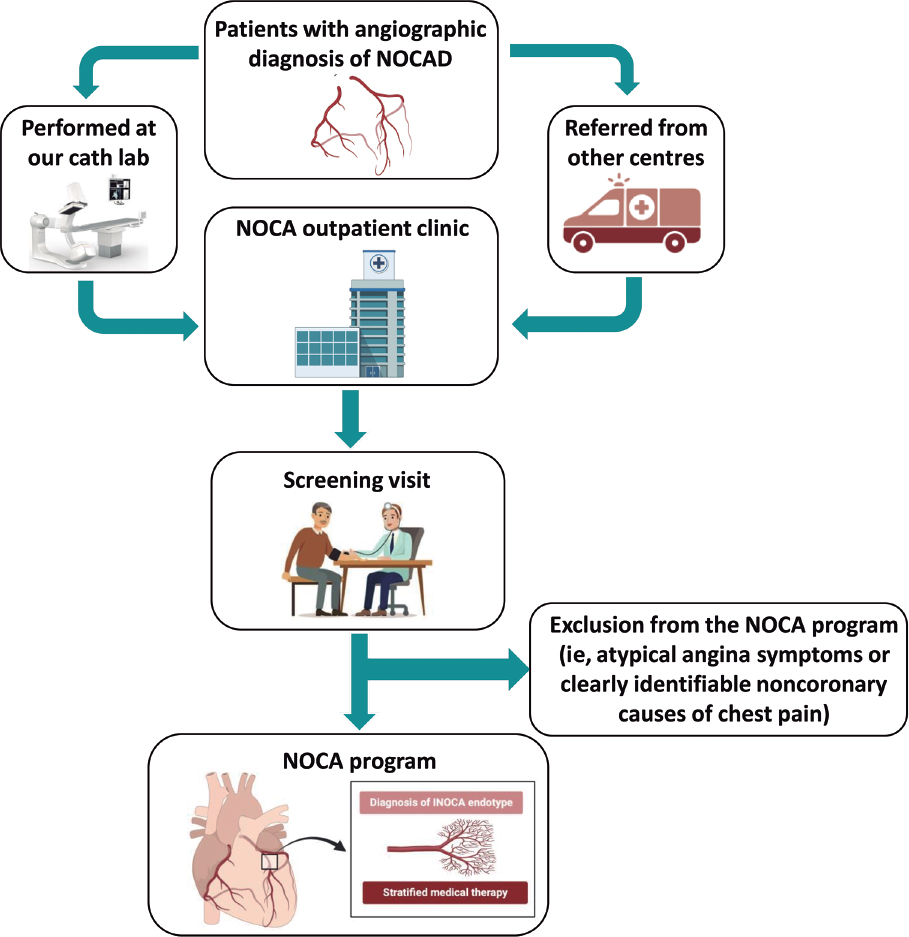
Figure 1. Central illustration. Flowchart for the inclusion of patients in the NOCA program. Cath lab, catheterization laboratory; INOCA: ischemia with no obstructed coronary arteries; NOCA, no obstructed coronary arteries; NOCAD, nonobstructive coronary artery disease.
NOCA program: diagnostic approach
After patient inclusion in the NOCA program, specialized counseling was provided by expert cardiologists and nurses. All patients were thoroughly informed about their disease, the importance of reaching a specific diagnosis, and the importance implementing tailored therapy. During the counseling sessions, the predicted benefits and low associated risks of an invasive procedure to specifically study coronary microcirculation and vasospasm were explained in detail. All patients provided written informed consent to undergo coronary angiography and intracoronary provocation testing with acetylcholine (ACh).
Subsequently, all patients underwent a scheduled coronary angiogram with a comprehensive diagnostic work-up consisting of the following: a) coronary function testing to assess coronary flow reserve (CFR) and the index of microvascular resistance (IMR); b) intracoronary ACh provocation testing to assess the presence of coronary vasomotion disorders (eg, epicardial or microvascular spasm).
Coronary function testing was performed using a pressure-temperature sensor guidewire (PressureWire X Guidewire and Coroventis CoroFlow Cardiovascular System, Abbott Vascular, United States) placed in the left anterior descending artery (LAD) as the prespecified target vessel, reflecting its subtended myocardial mass and coronary dominance. Steady-state hyperemia was induced using intravenous adenosine (140 µg/kg/min). If there was severe tortuosity of the LAD or evidence of myocardial ischemia in a region other than the territory of the LAD, the wire was placed in the right coronary artery or the left circumflex, as per the operator’s decision. CFR was calculated using thermodilution, defined as resting mean transit time divided by hyperemic mean transit time (abnormal CFR was defined as ≤ 2.5). IMR was calculated as the product of distal coronary pressure at maximal hyperemia multiplied by the hyperemic mean transit time (normal value < 25).6,9
Intracoronary ACh provocation testing was performed with a standardized protocol involving serial ACh infusions for 20 seconds at increasing concentrations (2-20-100 µg in the left coronary artery with an interval of 2-3 minutes between each injection) with concomitant assessment of the patient’s symptoms, electrocardiogram documentation, and angiographic scans. Patients taking vasoactive drugs (eg, calcium channel blockers and nitrates) underwent a wash-out period of at least 48 hours before the provocative test.10,11,12 Epicardial coronary spasm was defined as the reproduction of chest pain and ischemic electrocardiogram changes in association with a reduction in coronary diameter ≥ 90% from baseline in any epicardial coronary artery segment.13 Microvascular spasm was diagnosed when typical ischemic ST-segment changes (deviation ≥ 1 mm) and angina developed in the absence of epicardial coronary constriction (< 90% diameter reduction).14
Subsequently, patients were stratified into 4 endotypes: a) microvascular angina (MVA) (evidence of coronary microvascular dysfunction [CMD] defined as any abnormal CFR [< 2.5], IMR [≥ 25], or microvascular spasm); b) vasospastic angina (VSA) (CFR ≥ 2.5, IMR < 25 and epicardial spasm); c) both MVA and VSA (evidence of CMD and epicardial spasm); and d) noncoronary chest pain (CFR ≥ 2.5 and IMR < 25, with neither microvascular nor epicardial spasm).6
Any complications occurring during the invasive diagnostic work-up were documented, including bradyarrhythmias, atrial fibrillation, ventricular tachycardia or fibrillation, coronary perforations, death from any cause, and any other complications.
NOCA program: pharmacological and psychological therapeutic approach
Once the endotype was identified, medical treatment for each patient was optimized accordingly (table 1). In patients with MVA, treatment with beta-blockers and calcium channel blockers (CCBs) was started or up-titrated. Ranolazine was added if angina symptoms were not fully controlled by beta-blockers and CCBs. In patients with VSA, treatment with nondihydropyridine CCBs and long-acting nitrates was started or up-titrated. In patients with both MVA and VSA, treatment with nondihydropyridine CCBs or beta-blockers was started or up-titrated. In patients with noncoronary chest pain, vasoactive drugs were discontinued unless clinically indicated for other reasons. Additionally, treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and statins was started or up-titrated in all patients. If a patient showed intolerance or had contraindications to a specific medication (eg, asthma for beta-blockers, perimalleolar edema for CCBs, severe bradycardia for both beta-blockers and CCBs), the treatment was tailored and modified accordingly.
Because stress is an important trigger factor for angina symptoms, all patients were also referred to a team of expert psychologists for psychological support.15
NOCA program: clinical outcome and quality of life evaluation
All patients were followed up at 1, 3, 6, and 12-months for treatment titration and assessment of clinical outcomes. At the time of coronary angiography (ie, baseline) and at the 3-month follow-up, all patients were administered the Seattle Angina Questionnaire (SAQ) and quality of life questionnaire (EuroQol-5D [EQ-5D]). The SAQ is a validated 19-item self-administered questionnaire that measures 5 dimensions of CAD: physical limitation, angina stability, angina frequency, treatment satisfaction, and disease perception.16 The EQ-5D is a standardized, nondisease-specific questionnaire used to describe and evaluate patients’ health status and was intended to complement other quality-of life measures.17 Figure 2 provides a visual representation of all the steps involved for patients included in the NOCA program.
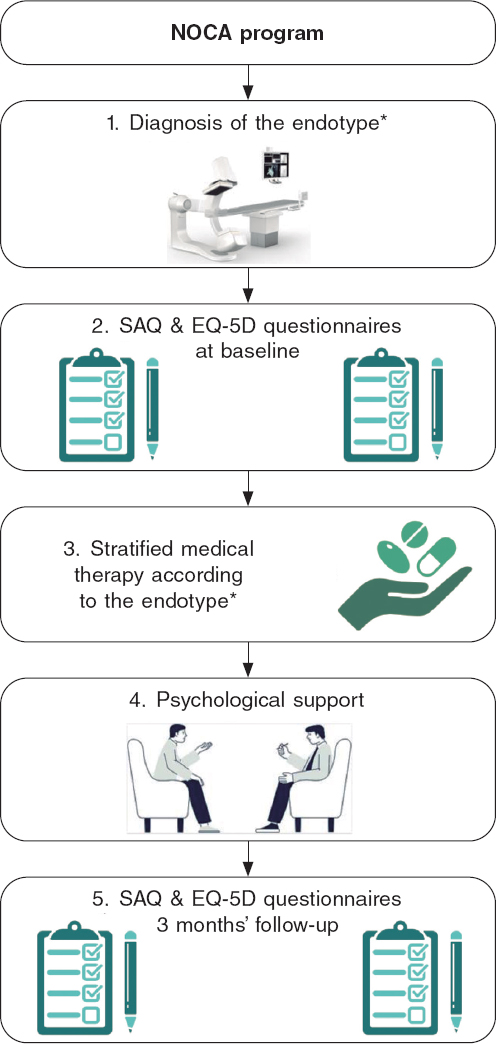
Figure 2. Visual representation of the NOCA program. EQ-5D, EuroQol-5D; NOCA, no obstructed coronary arteries; SAQ, Seattle Angina Questionnaire. * See text for more details.
Statistical analysis
Data distribution was assessed according to the Kolgormonov-Smirnov test. Continuous variables were compared using the unpaired Student t-test or the Mann–Whitney U test, as appropriate. The data are expressed as mean ± standard deviation (SD) or as median and interquartile range [IQR]. Categorical data are expressed as numbers and percentages and were evaluated using the chi-square test or Fisher exact test, as appropriate. A 2-sided P value < .05 was considered significant. All analyses were performed using SPSS version 21 (SPSS, United States).
RESULTS
Baseline characteristics of the study population
From January 2021 to December 2021, a total of 77 patients were screened at the NOCA clinic for inclusion in the NOCA program. Following the screening visit, 23 (29.9%) patients were excluded from the NOCA program: 12 due to atypical angina symptoms and 11 due to a clearly identifiable noncoronary cause. Consequently, 54 patients were included in the NOCA program (mean age 64.4 ± 9.4 years, 39 [63.9%] women). A total of 29 (53.7%) patients had INOCA and 25 (46.3%) had ANOCA. All clinical and angiographic characteristics of the study population are shown in table 1.
Table 1. Medical therapy according to the specific endotype of ANOCA/INOCA
| Pathogenic mechanism of MINOCA | Therapeutic implications |
|---|---|
| MVA | Beta-blockers (Nebivolol 2.5–10 mg daily) |
| CCBs (amlodipine 10 mg daily, or verapamil 240 mg daily, or diltiazem 90 mg twice daily) | |
| Ranolazine (375-750 mg twice daily) | |
| VSA | Nondihydropyridine CCBs (verapamil 240 mg, or ciltiazem 90 mg twice daily) |
| Long-acting nitrates (isosorbide mononitrate 30 mg) | |
| MVA and VSA | CCBs (verapamil or diltiazem) or beta-blockers |
| Noncoronary chest pain | Beta-blockers or dihydropyridine CCBs if clinically indicated (eg, hypertension) |
| ACEi or ARB if clinically indicated | |
| Statins if clinically indicated | |
ACEi, angiotensin-converting enzyme inhibitors; ANOCA, angina with no obstructed coronary arteries; ARB, angiotensin receptor blockers; CCBs, calcium channel blockers; INOCA, ischemia with no obstructed coronary arteries; MINOCA, myocardial infarction with non-obstructive coronary artery disease; MVA, microvascular angina; VSA, vasospastic angina. | |
NOCA program: diagnosis of the specific endotype and complications
The results of the invasive functional assessment are presented in table 2. The mean IMR and CFR values were 21.2 ± 10.6 and 2.3 ± 1.4, respectively. MVA was diagnosed in 19 (35.2%) patients, VSA in 12 (22.2%), and both MVA and VSA in 18 (33.3%). Finally, 5 (9.3%) patients were diagnosed with noncoronary chest pain.
Table 2. Clinical and angiographic characteristics of patients included in the NOCA program
| Characteristics | Study population (n = 54) |
|---|---|
| Clinical characteristics | |
| Age | 64.4 ± 9.4 |
| Female sex | 39 (72.2) |
| Clinical presentation | |
| ANOCA | 25 (46.3) |
| INOCA | 29 (53.7) |
| Diabetes mellitus | 12 (22.2) |
| Hypertension | 35 (64.8) |
| Dyslipidaemia | 28 (51.9) |
| Former smokers | 3 (5.7) |
| Current smoker | 14 (25.9) |
| Familiar history of CV disease | 5 (9.3) |
| Previous CV history | |
| Prior MI | 7 (13.0) |
| Prior PCI | 8 (14.8) |
| Prior CABG | 0 (0.0) |
| COPD | 1 (1.9) |
| CKD (eGFR < 60 mL/min/m2) | 4 (7.4) |
| Depression | 15 (27.8) |
| Anxiety | 19 (35.2) |
| Invasive functional evaluation | |
| Vessel explored | |
| LDA | 48 (88.9) |
| LCx | 3 (5.6) |
| RCA | 3 (5.6) |
| IMR | 21.2 ± 10.6 |
| Increased IMR (≥ 25) | 18 (33.3) |
| CFR | 2.3 ± 1.4 |
| Reduced CFR (< 2.5) | 33 (61.1) |
| Increased IMR (≥ 25) and reduced CFR (< 2.5) | 13 (24.1) |
| Diagnosis (endotype) | |
| MVA | 19 (35.2) |
| VSA | 12 (22.2) |
| MVA and VSA | 18 (33.3) |
| Noncoronary chest pain | 5 (9.3) |
ANOCA, angina with no obstructed coronary arteries; CABG, coronary artery bypass graft surgery; CFR, coronary flow reserve; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; IMR, index of microcirculatory resistance; INOCA, ischemia with no obstructed coronary arteries; MI, myocardial infarction; MVA, microvascular angina; LAD, left anterior descending; LCx, left circumflex; PCI, percutaneous coronary intervention; RCA, right coronary artery; VSA, vasospastic angina. Values are expressed as No. (%), mean ± standard deviation or median [interquartile range]. | |
Among INOCA patients, MVA was diagnosed in 11 (37.9%) patients, VSA in 7 (24.1%), both MVA and VSA in 8 (27.6%), and noncoronary chest pain in 3 (10.3%). Among ANOCA patients, MVA was diagnosed in 8 (32.0%) patients, VSA in 5 (20.0%), both MVA and VSA in 10 (40.0%), and noncoronary chest pain in 2 (8.0%). There were no statistically significant differences in the prevalence of any endotype between INOCA and ANOCA patients (all P > .05, figure 3).
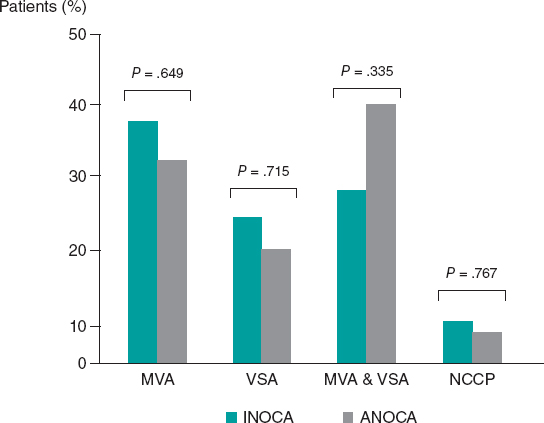
Figure 3. Prevalence of the different endotypes among INOCA and ANOCA patients. ANOCA, angina with no obstructed coronary arteries; INOCA, ischemia with no obstructed coronary arteries; MVA, microvascular angina; NCCP, noncoronary chest pain; VSA, vasospastic angina.
Complications occurred in 3 (5.5%) patients during intracoronary ACh provocation testing: 2 (3.7%) patients had transient bradyarrhythmias and 1 (1.8%) patient had paroxysmal atrial fibrillation that spontaneously reverted to sinus rhythm.
NOCA program: treatment optimization according to the specific endotype
Inclusion in the NOCA program led to statistically significant changes in medications after diagnosis of the specific endotype. There was a significant increase in the use of beta-blockers (33.3% before vs 57.4% after, P = .008), nondihydropyridine CCBs (9.3% before vs 37.0% after, P < .001), and long-acting nitrates (46.3% before vs 63.0% after, P = .012). There were no statistically significant differences in any other medications before and after the invasive assessment (all P > .05, figure 4). All changes in medications according to the specific endotype of ANOCA/INOCA are shown in figure 5.
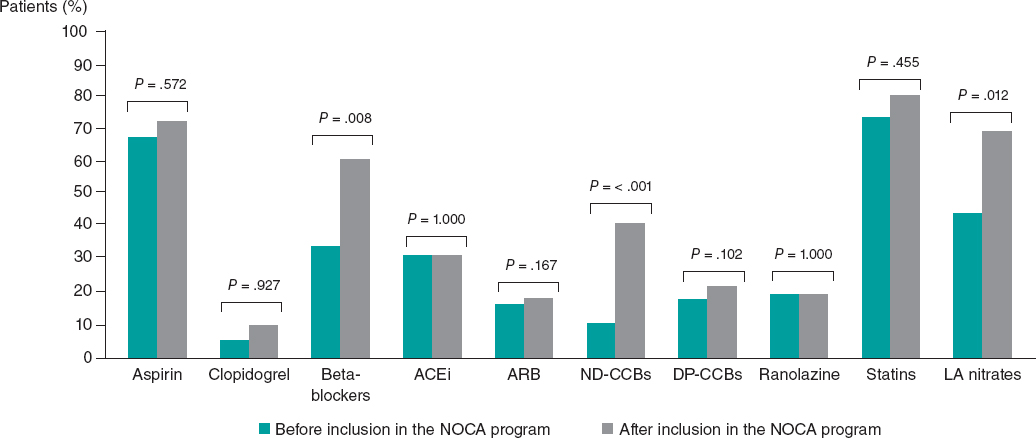
Figure 4. Differences in medical treatment before and after patient inclusion in the NOCA program. ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; DP-CCBs, dihydropyridine calcium channel blockers; LA, long-acting; ND-CCBs, nondihydropyridine calcium channel blockers.
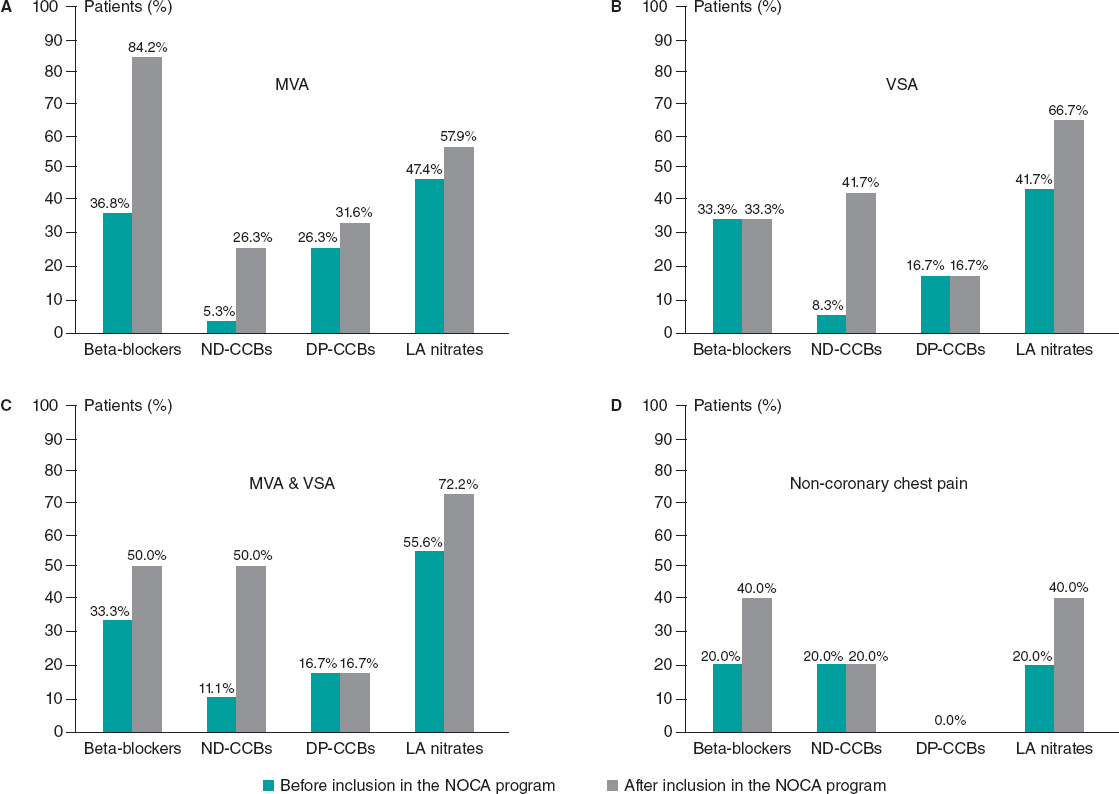
Figure 5. Changes in medications according to the specific endotype of ANOCA/INOCA. A: patients with MVA. B: patients with VSA. C: Patients with MVA and VSA. D: patients with noncoronary chest pain. DP-CCBs, dihydropyridine calcium channel blockers; LA, long-acting; MVA, microvascular angina; ND-CCBs, nondihydropyridine calcium channel blockers; VSA, vasospastic angina.
NOCA program: clinical outcome evaluation
At 3 months of follow-up, there was no statistically significant difference in the mean EQ-5D score compared with baseline (64.8 ± 18.1 at baseline vs 66.1 ± 17.1 at 3 months of follow-up, P = .302) (figure 6). However, there was a statistically significant improvement in the SAQ score in terms of physical limitations (59.7 ± 19.3 at baseline vs 66.2 ± 16.9 at 3 months of follow-up, P = .037), angina stability (57.1 ± 28.1 at baseline vs 75.8 ± 22.3 at 3 months of follow-up, P = .010), and disease perception (42.5 ± 13.9 at baseline vs 50.8 ± 16.3 at 3 months follow-up, P = .015). No statistically significant difference was found in angina frequency (74.3 ± 20.4 at baseline vs 80.7 ± 19.8 at 3 months of follow-up, P = .193) or treatment satisfaction (68.1 ± 12.6 at baseline vs 70.5 ± 12.5 at 3 months of follow-up, P = .950) (figure 7). No events were recorded at the 1-year follow-up.
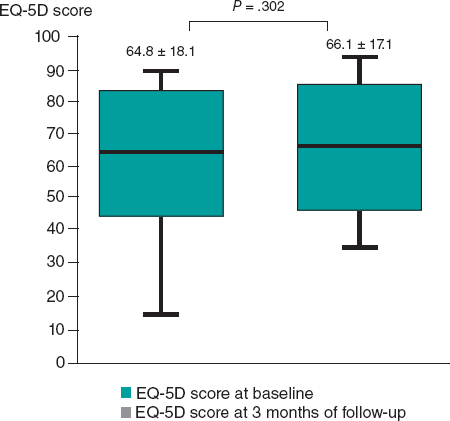
Figure 6. Differences in EuroQol-5D (EQ-5D) score at baseline and at 3 months of follow-up.
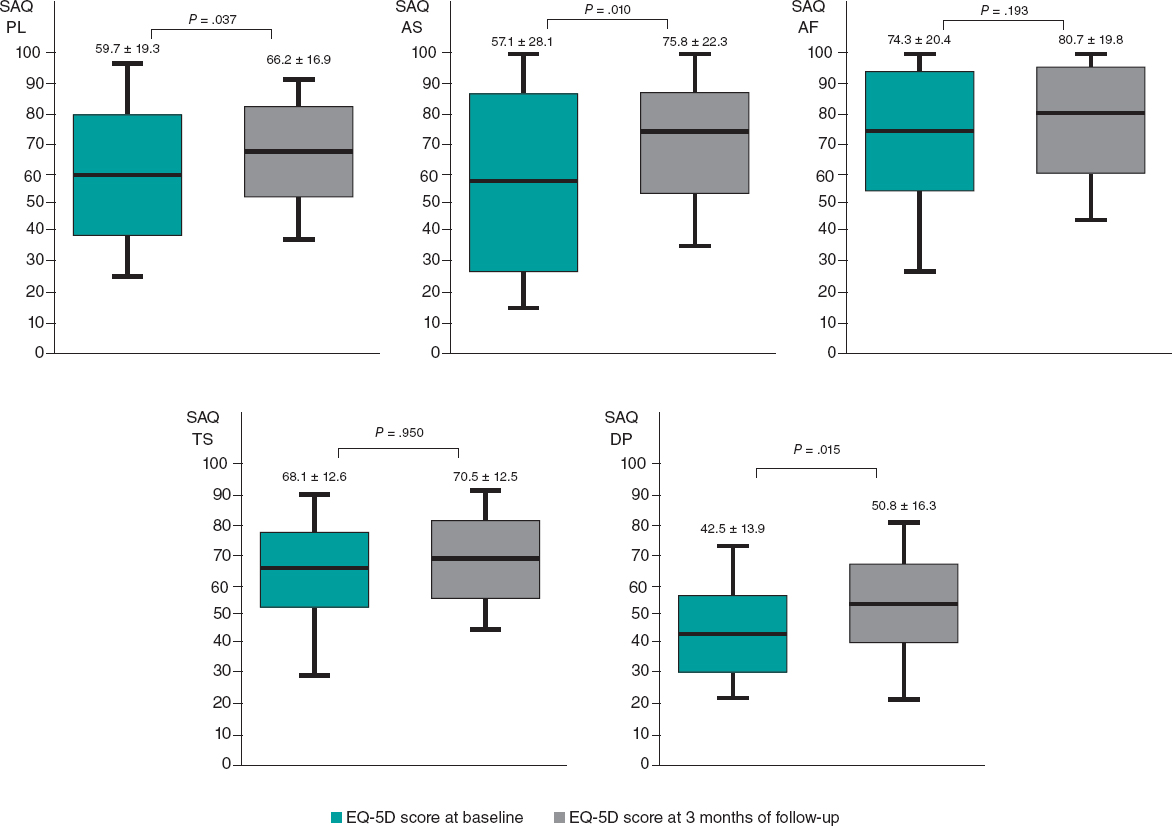
Figure 7. Differences in SAQ score at baseline and at 3 months of follow-up. AF, angina frequency; AS, angina stability; DP, disease perception; EQ-5D, EuroQol-5D; PL, physical limitations; SAQ, Seattle Angina Questionnaire; TS, treatment satisfaction.
DISCUSSION
The main results of our experience can be summarized as follows: a) the implementation of a specific diagnostic and therapeutic protocol (NOCA program) in patients with diagnosed with nonobstructive CAD is feasible and allowed a parsimonious use of medical resources; b) a comprehensive diagnostic work-up in INOCA and ANOCA patients is safe, with a low rate of mild and transient complications (5.5%); c) the inclusion of patients in the NOCA program led to significant changes in medications and a significant improvement in their angina symptoms at the 3-month follow-up with no adverse events at 1 year.
Although accumulating evidence has demonstrated that an approach consisting of a comprehensive diagnostic assessment and stratified medical therapy in INOCA and ANOCA is crucial to improve patients’ prognosis, such an approach is far from routinely implemented in clinical practice.7,8 There are still concerns mainly related to the cost-benefit ratio, the associated prolonged procedural time, increased costs, and the risk of possible associated complications. Furthermore, in the most recent European Society of Cardiology guidelines, invasive coronary function testing is assigned a class IIa (“should be considered”) recommendation, while ACh provocation testing is supported by a class IIb recommendation (“may be considered”) to assess microvascular spasm and class IIa in patients under consideration for VSA.3 As a result, the management of these patients is commonly left to physicians’ discretion or relies on the experience of each center. Consequently, diagnosis of a specific NOCA endotype is frequently missed, and medical therapy is not optimized. This, in turn, has a significant negative impact on patients’ quality of life and clinical outcomes, as well as on health care costs, due to the need for repeat hospitalization or invasive procedures.18
In reporting our experience, we demonstrate that a specific diagnostic and therapeutic protocol (ie, the NOCA program) in patients with a previous diagnosis of nonobstructive CAD can be easily implemented in clinical practice. A key innovation of our study, compared with prior publications, is the creation and implementation of a specific protocol for the INOCA/ANOCA population. Additionally, our approach involves a screening visit with assessment by a team of expert cardiologists for patients with a suspected diagnosis of INOCA/ANOCA. This approach improves identification of such patients, and, in our experience, led to the exclusion of almost one third of patients (29.9%) due to atypical angina symptoms or no clearly identifiable coronary causes of chest pain. This is another novelty of our study that could be extremely relevant in the management of these patients. Indeed, the selection of patients to be included in the program may allow clinical resources to be directed to patients who are most likely to benefit, while avoiding repeat invasive procedures and related risks in patients with unclear indications. Additionally, the specialized counseling provided by cardiologists and nurses during the screening visit, together with psychological support, are likely to be vital components of the management of INOCA/ANOCA patients. Indeed, recent studies have demonstrated how psychological factors, such as chronic stress, anxiety, depression, and social stressors are involved in the pathogenesis of MVA and VSA.19-23 Mental stress has been demonstrated to determine CMD mainly through endothelium-dependent mechanisms and endothelial dysfunction.24 Similarly, by activating brain areas involved in regulation of neuroendocrine and autonomic nervous systems, mental stress can lead to hyperreactivity of vascular smooth muscle cells, autonomic nervous system dysfunction, oxidative stress, vascular inflammation, and endothelial dysfunction, resulting in an increased propensity to coronary vasospasm.25-27
Furthermore, in line with previous studies,28-30 our experience demonstrates that performing a comprehensive invasive diagnostic assessment for the diagnosis of the specific endotype in INOCA and ANOCA patients is safe and is associated with a low rate of mild and transient complications. For all these reasons, patients and clinicians should be reassured about the lack of serious complications and cardiologists should be strongly encouraged to implement a specific diagnostic and therapeutic program in these patients. Indeed, the availability of such a program for INOCA and ANOCA patients may have significant clinical and therapeutic implications, as, in our experience, it resulted in substantial changes in medications and a marked improvement at the 3-month follow-up of the SAQ questionnaire regarding physical limitations, angina frequency, and disease perception. The lower and nonsignificant improvement in the other parameters (eg, angina frequency and treatment satisfaction) could be attributed to the already high baseline values (74.5 ± 19.9 and 69.6 ± 11.9, respectively). Similarly, the absence of a significant improvement in the EQ-5D questionnaire at 3 months might be due to the short follow-up period or the fact that it is a nondisease-specific questionnaire designed to describe and assess patients’ health status and is intended to complement other quality-of-life measures.31
Study limitations
Some limitations of this study should be acknowledged. First, this is a single-center study with a relatively small sample size and short follow-up. Second, we did not perform a cost-analysis and therefore we cannot speculate on the impact of the NOCA program on health care-related costs. Further studies in larger ANOCA and INOCA populations are warranted. Finally, the absence of a control group precluded a thorough assessment of the improvement in the quality of life among these patients.
CONCLUSIONS
Our experience demonstrates that a specific diagnostic and therapeutic protocol (NOCA program) can be easily and safely implemented in routine clinical practice. Such a protocol could ensure the best care for INOCA and ANOCA patients, as well as improve their quality of life and avoid inappropriate treatments and incomplete investigations. Future evidence from randomized clinical trials or recommendations from international clinical guidelines supporting the implementation of a specific protocol in these patients are strongly warranted.
FUNDING
This study received no funding.
ETHICAL CONSIDERATIONS
The study protocol complied with the Declaration of Helsinki and the study was approved by our Institutional Review Committee. All patients gave written informed consent to be included in this program and study. The clinical ethics committee gave their approval for a retrospective analysis of the data collected. In this work, the possible variables of sex and gender have been taken into account.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used during the preparation of this work.
AUTHORS’ CONTRIBUTIONS
R. Rinaldi, F. Spione, F.M. Verardi: data extraction and analysis and manuscript drafting; R. Rinaldi, F. Spione, S. Brugaletta: design and manuscript revision; P. Vidal Calés, V. Arévalos, R. Gabani, D. Cánovas, M. Gutiérrez, M. Pardo, R. Domínguez, L. Pintor, X. Torres, X. Freixa, A. Regueiro, O. Abdul-Jawad Altisent, M. Sabaté: manuscript revision. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
ACKNOWLEDGMENTS
F. Spione has been supported by a research grant provided by the Cardiopath PhD program.z
WHAT IS KNOWN ABOUT THE TOPIC?
- Up to 60% to 70% of patients with angina and/or documented myocardial ischemia do not have angiographic evidence of obstructive coronary artery disease. This condition is defined as angina with no obstructed coronary arteries (ANOCA) or ischemia with no obstructed coronary arteries (INOCA) when associated with evidence of myocardial ischaemia. There are still concerns about the implementation in real practice of a systematic diagnostic and therapeutic approach in INOCA and ANOCA patients, potentially impacting outcomes and quality of life.
WHAT DOES THIS STUDY ADD?
- The implementation of a specific protocol (NOCA program) in patients with a diagnosis of nonobstructive CAD is feasible and allowed parsimonious use of medical resources. A comprehensive invasive diagnostic assessment in INOCA or ANOCA patients is safe and is associated with a low rate of mild and transient complications. The availability of a specific diagnostic and therapeutic program for INOCA and ANOCA patients may have important clinical and therapeutic implications, as, in our experience, it led to significant changes in medications and a notable improvement at 3 months of follow-up in the SAQ questionnaire regarding physical limitations, angina frequency, and perception of the disease.
REFERENCES
1. Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019:Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982-3021.
2. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886-895.
3. Neumann FJ, Sechtem U, Banning AP, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
4. Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734-744.
5. Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840-849.
6. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology &Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
7. Ford TJ, Stanley B, Good R, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina:The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841-2855.
8. Ford TJ, Stanley B, Sidik N, et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc Interv. 2020;13:33-45.
9. Candreva A, Gallinoro E, van 't Veer M, et al. Basics of Coronary Thermodilution. JACC Cardiovasc Interv. 2021;14:595-605.
10. Montone RA, Meucci MC, De Vita A, Lanza GA, Niccoli G. Coronary provocative tests in the catheterization laboratory:Pathophysiological bases, methodological considerations and clinical implications. Atherosclerosis. 2021;318:14-21.
11. Ford TJ, Ong P, Sechtem U, et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease:Why, How, and When. JACC Cardiovasc Interv. 2020;13:1847-1864.
12. Gutiérrez E, Gómez-Lara J, Escaned J, et al. Assessment of the endothelial function and spasm provocation test performed by intracoronary infusion of acetylcholine. Technical report from the ACI-SEC;REC Interv Cardiol. 2021;3:286-296.
13. Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565-2568.
14. Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20.
15. Jespersen L, Abildstrøm SZ, Hvelplund A, Prescott E. Persistent angina:highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571-581.
16. Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7:640-647.
17. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
18. Jespersen L, Abildstrom SZ, Hvelplund A, et al. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease:a registry-based cohort study. PLoS One. 2014;9:e93170.
19. Smaardijk VR, Lodder P, Kop WJ, van Gennep B, Maas AHEM, Mommersteeg PMC. Sex- and Gender-Stratified Risks of Psychological Factors for Incident Ischemic Heart Disease:Systematic Review and Meta-Analysis. J Am Heart Assoc. 2019;8:e010859.
20. Mehta PK, Hermel M, Nelson MD, et al. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction:Results from the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) study. Int J Cardiol. 2018;251:8-13.
21. Konst RE, Elias-Smale SE, Lier A, Bode C, Maas AHEM. Different cardiovascular risk factors and psychosocial burden in symptomatic women with and without obstructive coronary artery disease. Eur J Prev Cardiol. 2019;26:657-659.
22. Gomez MA, Merz NB, Eastwood JA, et al. Psychological stress, cardiac symptoms, and cardiovascular risk in women with suspected ischaemia but no obstructive coronary disease. Stress Health. 2020;36:264-273.
23. Bekendam MT, Vermeltfoort IAC, Kop WJ, Widdershoven JW, Mommersteeg PMC. Psychological factors of suspect coronary microvascular dysfunction in patients undergoing SPECT imaging. J Nucl Cardiol. 2022;29:768-778.
24. Hammadah M, Kim JH, Al Mheid I, et al. Coronary and Peripheral Vasomotor Responses to Mental Stress. J Am Heart Assoc. 2018;7:e008532.
25. Shah A, Chen C, Campanella C, et al. Brain correlates of stress-induced peripheral vasoconstriction in patients with cardiovascular disease. Psychophysiology. 2019;56:e13291.
26. Hung MY, Mao CT, Hung MJ, et al. Coronary Artery Spasm as Related to Anxiety and Depression:A Nationwide Population-Based Study. Psychosom Med. 2019;81:237-245.
27. Crea F, Montone RA, Rinaldi R. Pathophysiology of Coronary Microvascular Dysfunction. Circ J. 2022;86:1319-1328.
28. Probst S, Seitz A, Martínez Pereyra V, et al. Safety assessment and results of coronary spasm provocation testing in patients with myocardial infarction with unobstructed coronary arteries compared with patients with stable angina and unobstructed coronary arteries. Eur Heart J Acute Cardiovasc Care.2020:2048∖20932422.
29. Montone RA, Rinaldi R, Del Buono MG, et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and nonobstructive coronary arteries. EuroIntervention 2022;18:e666-e676.
30. Rinaldi R, Salzillo C, CaffèA, Montone RA. Invasive Functional Coronary Assessment in Myocardial Ischemia with Non-Obstructive Coronary Arteries:from Pathophysiological Mechanisms to Clinical Implications. Rev Cardiovasc Med. 2022;23:371.
31. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199-208.











