Simply stated, the goal of diagnostic coronary angiography is to distinguish the cause of a patient’s chest pain from 1 of 4 endotypes: a) epicardial stenosis; b) coronary spasm; c) coronary microvascular disease (CMD); and d) —equally important—noncoronary chest pain. Crucially, the latter is a diagnosis of exclusion and consequently cannot be confirmed without formal assessment of the other mechanisms (figure 1). Despite this truism, the interpretation of most coronary angiograms is limited to simple “eyeballing” of an epicardial “shadowgram”. This approach has a low diagnostic yield with 40% of patients found to have no significant epicardial stenoses—an entity known as angina with no obstructive coronary arteries (ANOCA).1 Despite the presence of typical angina or evidence of ischemia during noninvasive testing, these patients, are frequently nonchalantly dismissed without a formal diagnosis.
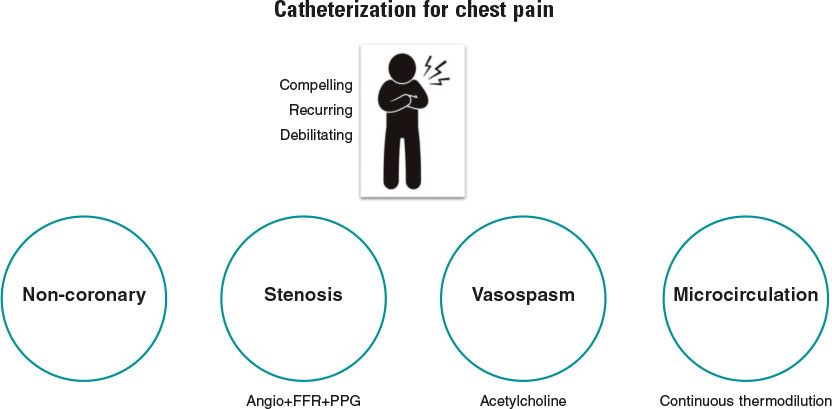
Figure 1. Patients with compelling, recurring, and debilitating chest pain should undergo catheterization with coronary angiography and—when needed—coronary function testing to unravel the mechanism of their pain. Noncoronary chest pain is a diagnosis of exclusion and consequently can only be confirmed if the 3 other mechanisms have been assessed. FFR, fractional flow reserve; PPG, pullback pressure gradient.
This very large group of patients is heterogeneous, and establishing the underlying cause of ANOCA requires a thorough coronary function testing (CFT) protocol that includes diagnostic angiography, provocation testing for microvascular or epicardial vasospasm, and assessment of CMD.2 In many centers, however, diagnostic angiography is rarely complemented with CFT. Among those that do, testing is often incomplete, with the result that patients often do not receive a diagnosis of the underlying cause of their ANOCA or benefit from potential endotype-specific treatments. Possible explanations for this behavior include a lack of familiarity with the causes of ANOCA, a lack of knowledge of available testing modalities, concerns about the accuracy of tests, and a belief that the underlying diseases are untreatable.
In a recent article published in REC: Interventional Cardiology, Rinaldi et al.3 describe their single-center experience of the implementation of a specific ANOCA diagnostic and therapeutic protocol at Hospital Clínic in Barcelona, Spain. In this program, all patients with ANOCA underwent systematic CFT including bolus thermodilution for the calculation of coronary flow reserve and the index of microvascular resistance, as well as intracoronary provocation testing to assess epicardial or microvascular spasm. Based on the results of these tests, patients were classified into 4 endotypes: a) microvascular angina (MVA) (CMD or microvascular spasm); b) vasospastic angina (epicardial spasm); c) both MVA and vasospastic angina; and d) noncoronary chest pain.
The authors demonstrated that, as a result of the identification of specific ANOCA endotypes, there were significant increases in targeted medical prescriptions such as beta-blockers, nondihydropyridine calcium channel blockers, and long-acting nitrates. While this did not translate into a statistically significant improvement in quality of life between baseline and 3 months, angina significantly improved in terms of physical limitation, angina stability, and disease perception. Importantly, the protocol was shown to be safe, with only 3 minor adverse events being reported, all occurring during acetylcholine administration (transient bradycardia, paroxysmal atrial fibrillation with spontaneous cardioversion).
This work has significant strengths that should be highlighted. The CorMicA trial provided the first evidence from a randomized controlled trial of the benefits of systematic CFT with targeted medical therapy.4 However, to date, scarce real-world data have been available on the implementation of such protocols in routine clinical practice. As such, this small, real-world, observational study is strongly welcomed. More than the clinical results obtained in a relatively small number of patients, the work by Rinaldi et al.3 is particularly worthwhile for several methodological aspects, 3 of which are discussed below.
(i) Which patients should enter such a program and how? Patients were screened at a specific outpatient clinic and their inclusion was based on well-standardized criteria. Ideally, only patients with compelling, recurrent and invalidating symptoms should undergo CFT. The usefulness of such a program is significantly reduced by the referral of patients with unconvincing symptoms, or those with a high pretest probability of epicardial disease. Notably, the increasing role of coronary computed tomography angiography for the screening of epicardial disease in patients with angina currently allows diagnosis of ANOCA without the use of invasive diagnostic angiography and patients can thus be referred directly for CFT.
(ii) How should CFT be performed? As described by the authors, both microvascular function and coronary vasomotion should be investigated in a strictly standardized manner, preferably in the left anterior descending artery. However, the order of these tests is debatable. In our opinion, it does not make sense to investigate endothelial function and coronary vasomotion with a guidewire in the coronary artery or when the patient has already received vasoactive medications such as nitrates and calcium channel blockers. Consequently, we believe that acetylcholine testing should come first, with epicardial vasodilation being induced with nitrates at the end of acetylcholine testing. The latter also represents an important step (and good clinical practice) before commencing wire-based measurements of microcirculatory function. As for the choice of testing modality, bolus thermodilution should be replaced by continuous thermodilution as it enables the calculation of coronary flow reserve, absolute microvascular resistance (Rµ) and microvascular resistance reserve from absolute volumetric flow rather than from surrogates of flow.5 Future studies are expected to clarify the clinically relevant cutoff values for the indices derived from continuous thermodilution. These values should allow a more robust definition of CMD, which is currently an unmet need.
(iii) How should these patients be followed up? Patients should be followed up by the same physicians in a dedicated outpatient clinic, and be asked about their symptoms in a structured and systematic way. In this regard, the authors should be commended for the use of structured questionnaires to assess angina and quality of life. The classic Canadian Cardiovascular Society grading system for angina is not appropriate for ANOCA patients as their symptoms often differ from those reported by patients with epicardial disease. Moreover, objective signs of ischemia are often lacking. In the future, it is likely that patients will be given an app on their smartphone to closely monitor their symptoms.6
Overall, as with any new program, it is important to recognise that there is a learning phase. However, with a well-structured and standardized program, such as that proposed by Rinaldi et al.,3 this learning phase is likely to be short. There is now a need for future work addressing the implications of such a protocol on both time and cost in routine clinical practice. For example, in the setting of a busy, real-world catheterization laboratory, how much time, on average, does such a protocol add to the length of the procedure? Furthermore, what are the cost implications, and are they sufficiently counterbalanced by an increase in quality of life and/or symptom control?
To conclude, there are now strong clinical grounds for the systematic implementation of CFT in ANOCA patients. Furthermore, as demonstrated by Rinaldi et al., a standardized and robust testing program can be effectively implemented in real-world practice.3 Validation of clear diagnostic criteria for CMD is now needed for the results of CFT to be easily interpreted and acted upon. Patients and their referring clinicians deserve nothing less.
FUNDING
No funding was received to assist with the preparation of this editorial.
CONFLICTS OF INTEREST
T. Mahendiran is supported by a grant from the Swiss National Science Foundation (SNSF). B. De Bruyne has a consulting relationship with Boston Scientific, Abbott Vascular, CathWorks, Siemens, and Coroventis Research; receives research grants from Abbott Vascular, Coroventis Research, Cathworks, Boston Scientific; and holds minor equities in Philips-Volcano, Siemens, GE Healthcare, Edwards Life Sciences, HeartFlow, Opsens, Sanofi, and Celyad.
REFERENCES
1. Patel MR, Peterson ED, Dai D, et al. Low Diagnostic Yield of Elective Coronary Angiography. N Engl J Med. 2010;362:886-895.
2. Jansen TPJ, Konst RE, Elias-Smale SE, et al. Assessing Microvascular Dysfunction in Angina With Unobstructed Coronary Arteries. J Am Coll Cardiol. 2021;78:1471-1479.
3. Rinaldi R, Spione F, Verardi FM, et al. Angina or ischemia with no obstructed coronary arteries:a specific diagnostic and therapeutic protocol. REC Interv Cardiol. 2023. https://doi.org/10.24875/RECICE.M23000418.
4. Ford TJ, Stanley B, Good R, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina:The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841-2855.
5. De Bruyne B, Pijls NHJ, Gallinoro E, et al. Microvascular Resistance Reserve for Assessment of Coronary Microvascular Function:JACC Technology Corner. J Am Coll Cardiol. 2021;78:1541-1549.
6. Nowbar AN, Howard JP, Shun-Shin MJ, et al. Daily angina documentation versus subsequent recall:development of a symptom smartphone app. Eur Heart J Digit Health. 2022;3:276-283.
* Corresponding author.
E-mail address: Bernard.de.bruyne@olvz-aalst.be (B. De Bruyne).











