Percutaneous coronary interventions with drug-eluting stent (DES) implantation have become a well-established treatment for obstructive coronary artery disease, improving long-term outcomes.1 However, despite recent improvements including thinner strut platforms and more biocompatible polymers, the Achilles’ heel of DES strategy remains the risk of DES-related adverse events such as in-stent restenosis or stent thrombosis in the short term,2 along with an increase in hard clinical events at a rate of 2.0 to 3.5% yearly after the first year.3,4
Drug-coated balloons (DCB) have been developed as an alternative to percutaneous coronary intervention with DES implantation in selected populations for the treatment of coronary artery disease. The main advantage of this technology is its ability to deliver an antiproliferative drug to the treated lesion without leaving any layer of metal, which might cause late adverse events. Another advantage is the potential reduction in the duration or discontinuation of dual antiplatelet therapy, especially in patients at high risk of bleeding.
Several studies have investigated the role of DCB in real-world patients, who are those mainly affected by in-stent restenosis or de novo small vessel disease.5-9 The only randomized study of DCB in de novo small vessels with a clinical primary endpoint was BASKET-SMALL-2. This study demonstrated the noninferiority of DCB vs DES (vessel size 2-3 mm), which was maintained up to 3 years follow-up in terms of all clinical endpoints.5
The initial fear of leaving behind a residual coronary dissection, especially in de novo lesions, could limit the widespread use of DCB. However, it has been shown that a nonflow-limiting dissection after DCB treatment tends to heal during the first few months, with both the paclitaxel and sirolimus technologies, without leading to acute or subacute vessel closure.10,11
The main message regarding DCB is that they should be used as the final step of percutaneous coronary intervention and only when a proper lesion preparation has been performed with a fully expanded balloon of the correct size for the vessel, with accurate management of calcifications and no residual stenosis greater than 30% that could impair drug delivery to the vessel and limit the potential of this technology.
Recently, a new generation of DCB eluting sirolimus (SCB, Magic Touch, Concept Medical, United States) has been introduced that uses nanoparticles composed of a dual layer of phospholipids encapsulating the antiproliferative agent. Histopathologic studies have demonstrated therapeutic concentrations of the drug within the vessel wall for up to 60 days after percutaneous coronary intervention.12
Notably, the angiographic performance of this class of drug seems to be inferior to that provided by paclitaxel. The recently published TRANSFORM I trial showed that SeQuent Please DCB (B. Braun, Germany) outperformed SCB in terms of angiographic parameters at 6 months of follow-up, but without showing any difference in clinical endpoints. This lower performance of SCB seems to occur particularly in cases of complex lesions, emphasizing the importance of adequate lesion preparation, especially with the less lipophilic drug sirolimus (figure 1).13 Somewhat reassuringly, the performance of SCB in terms of clinical endpoints has been demonstrated in all-comer populations, especially in the prospective EASTBOURNE study, which showed a good safety and efficacy profile up to 2 years of follow-up in 2123 patients/2440 lesions.14
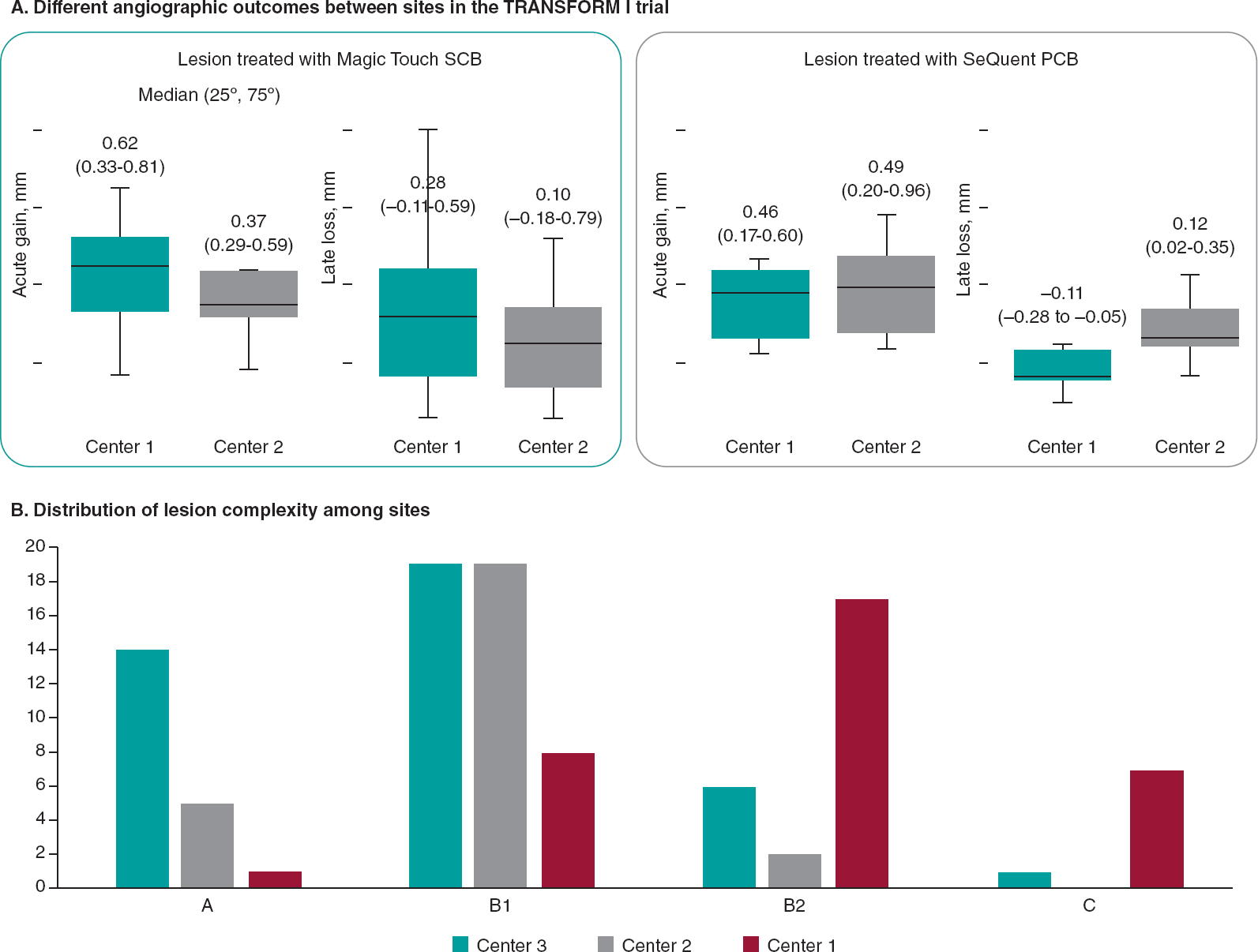
Figure 1. Differences in terms of types of lesion and outcomes among 2 top enroller centers for the TRANSFORM II trial. PCB, paclitaxel-coated balloon; SCB, sirolimus-coated balloon.
The next step to ensure wider use of this new generation DCB will be direct comparison with DES, as in the TRANSFORM II (NCT04893291) trial. This is an international, multicenter, prospective, investigator-driven, open-label, randomized (1:1) clinical trial designed to test the efficacy of SCB vs DES in native coronary artery vessels with diameters between 2.0 and 3.5 mm. Inclusion and randomization are being performed after adequate lesion preparation in the absence of flow-limiting dissection and acute vessel recoil. The study population has been calculated expecting the noninferiority of SCB in terms of target lesion failure at 12 months, and its sequential superiority in terms of net-adverse clinical events, including BARC 3-5 bleeding events. Interestingly, patients will be followed up clinically for 5 years to observe the potential superiority of DCB in the long-term. This trial, which includes 7 Spanish centers, is including patients at 40 centers allocated in 11 countries in Europe, Asia, and South America.15 By November 20th, 2023, 600 patients out of the planned 1820 had been enrolled.
The TRANSFORM II trial will be an essential test of the maturity of DCB in such an established, prognostically significant arena, challenging DES as the gold standard for the treatment of patients with native coronary artery disease.
FUNDING
None.
CONFLICTS OF INTEREST
B. Cortese serves on the advisory board or as a consultant for several companies producing or marketing DCB: Cordis, Medalliance, BBraun, Concept Medical, Medtronic, Innova HTS, and ANT.
REFERENCES
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
2. Sarno G, Lagerqvist B, Frobert O, et al. Lower risk of stent thrombosis and restenosis with unrestricted use of 'new-generation'drug-eluting stents:a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2012;33:606-613.
3. Kufner S, Ernst M, Cassese S, et al. 10-Year Outcomes From a Randomized Trial of Polymer-Free Versus Durable Polymer Drug-Eluting Coronary Stents. J Am Coll Cardiol. 2020;76:146-158.
4. Brugaletta S, Gomez-Lara J, Ortega-Paz L, et al. 10-Year Follow-Up of Patients With Everolimus-Eluting Versus Bare-Metal Stents After ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2021;77:1165-1178.
5. Jeger RV, Farah A, Ohlow MA, et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2):3-year follow-up of a randomised, non-inferiority trial. Lancet. 2020;396:1504-1510.
6. Giacoppo D, Alfonso F, Xu B, et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis:a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study). Eur Heart J. 2020;41:3715-3728.
7. Wanha W, Bil J, Januszek R, et al. Long-Term Outcomes Following Drug-Eluting Balloons Versus Thin-Strut Drug-Eluting Stents for Treatment of In-Stent Restenosis (DEB-Dragon-Registry). Circ Cardiovasc Interv. 2021;14:e010868.
8. Cortese B, Di Palma G, Guimaraes MG, et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease:PICCOLETO II Randomized Clinical Trial. JACC Cardiovasc Interv. 2020;13:2840-2849.
9. Cortese B, Testa G, Rivero F, Erriquez A, Alfonso F. Long-Term Outcome of Drug-Coated Balloon vs Drug-Eluting Stent for Small Coronary Vessels:PICCOLETO-II 3-Year Follow-Up. JACC Cardiovasc Interv. 2023;16:1054-1061.
10. Cortese B, Silva Orrego P, Agostoni P, et al. Effect of Drug-Coated Balloons in Native Coronary Artery Disease Left With a Dissection. JACC Cardiovasc Interv. 2015;8:2003-2009.
11. El Khoury A, Lazar L, Cortese B. The fate of coronary dissections left after sirolimus-coated balloon angioplasty:A prespecified subanalysis of the EASTBOURNE study. Catheter Cardiovasc Interv. 2023;102:979-986.
12. Cortese B, Kalkat H, Bathia G, Basavarajaiah S. The evolution and revolution of drug coated balloons in coronary angioplasty:An up-to-date review of literature data. Catheter Cardiovasc Interv. 2023;102:1069-1077.
13. Ono M, Kawashima H, Hara H, et al. A Prospective Multicenter Randomized Trial to Assess the Effectiveness of the MagicTouch Sirolimus-Coated Balloon in Small Vessels:Rationale and Design of the TRANSFORM I Trial. Cardiovasc Revasc Med. 2021;25:29-35.
14. Cortese B, Testa L, Heang TM, et al. Sirolimus-Coated Balloon in an All-Comer Population of Coronary Artery Disease Patients:The EASTBOURNE Prospective Registry. JACC Cardiovasc Interv. 2023;16:1794-1803.
15. Greco A, Sciahbasi A, Abizaid A, et al. Sirolimus-coated balloon versus everolimus-eluting stent in de novo coronary artery disease:Rationale and design of the TRANSFORM II randomized clinical trial. Catheter Cardiovasc Interv. 2022;100:544-552.











