HOW WOULD I APPROACH IT?
The case presented here is very representative of a certain complication that luckily, we rarely find nowadays: vessel acute occlusion following a coronary intervention. In this context, the use of intracoronary imaging modalities is essential to determine the mechanisms underlying this occlusion and to guide the treatment. Probably the most widely known mechanisms have to do with stent thrombosis such as the infra-expansion or dissection of stent edges, but in this case, there is a less common cause: the distal progression of a coronary intramural hematoma.
It could be argued if this iatrogenic intramural hematoma is related to the damage caused by the stent to the coronary wall, the powerful antiplatelet therapy used, etc., or whether a spontaneous hematoma is underlying the culprit lesion of the right coronary artery. Although the angiographic imaging is not typical of a dissection/spontaneous intramural hematoma, there are indistinguishable cases of type III atherosclerotic coronary lesions (figure 1) according to the classification established by Saw et al.1 that require the use of intracoronary imaging modalities to achieve diagnosis. The use of imaging modalities in culprit lesions of an acute coronary syndrome prior to the coronary intervention provides important information to guide the procedure and even change our therapeutic approach. For example, in this case and even though it can be controversial if it is confirmed that the cause is an spontaneous hematoma and taking into consideration the patient’s clinical stability, the absence of pain or ST-segment elevation and the presence of normal coronary flow, conservative therapy without stent implantation could be prescribed in an attempt to promote the vessel spontaneous healing.
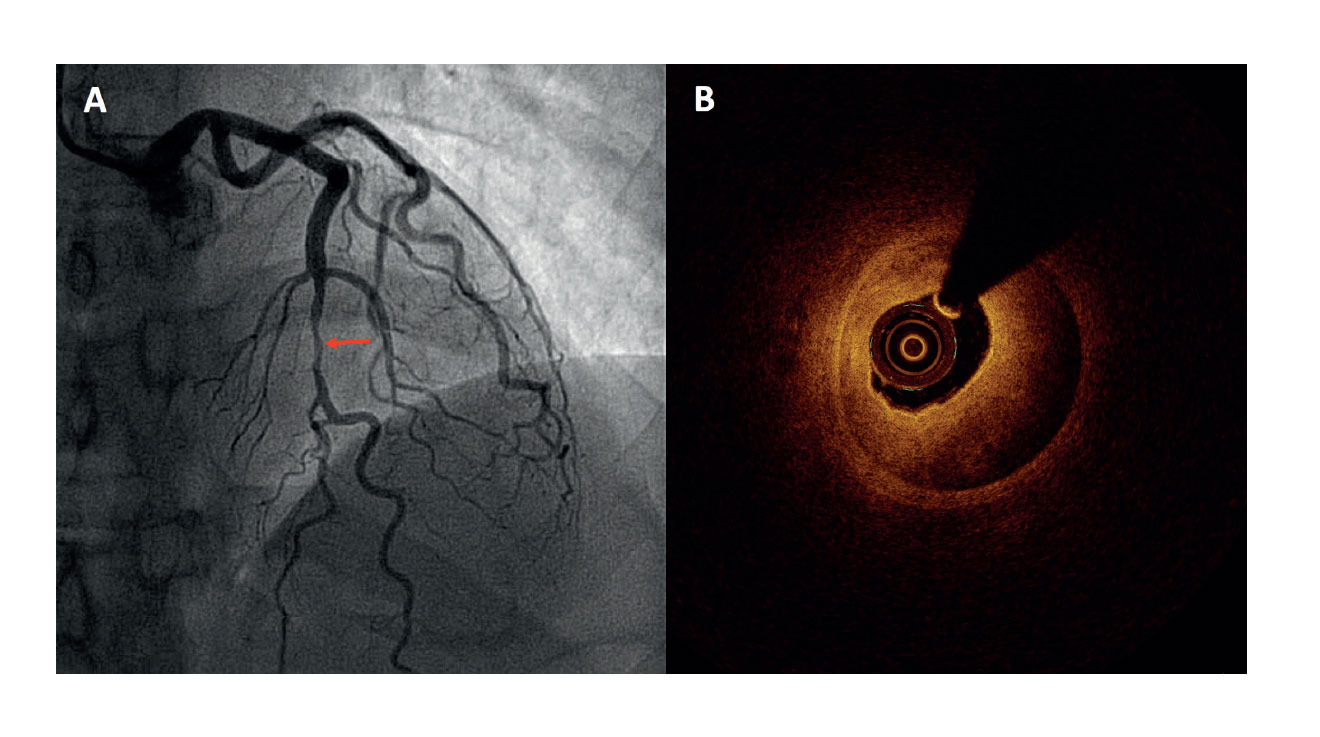
Figure 1. A: coronary angiography in a patient with acute coronary syndrome without ST-segment elevation showing one serious lesion in the mid anterior descending artery (arrow). B: the optical coherence tomography showed evidence of an intramural hematoma as the cause for such lesion.
Regarding the strategy of this case, I agree with the authors on the use of intravascular ultrasound (IVUS) as the imaging modality to use here since distal to the stent the vessel has a total occlusion that makes it difficult to perform an optical coherence tomography (OCT). As well as confirming the diagnosis of intramural hematoma, the IVUS would also allow us to evaluate its distal spreading, damage to lateral branches and, if coronary intervention is required, confirm the position of the guidewire inside the true lumen.
In this case it was decided to use a direct stent, which is probably the safest option to recover distal flow and prevent the re-occlusion of the vessel. However, some authors recommend the implantation of a stent only when flow cannot be restored using balloon dilatation. There are several reasons for this: in the first place, it is common that the stent causes the distal or proximal progression of the hematoma or dissection that may require multiple stents, occlude the lateral branches and, at times, lead to high-risk situations such as progression towards the left main coronary artery. Also, if an appropriate distal flow is finally recovered using balloon dilatation and myocardial ischemia is prevented, the healing of the vessel has been reported in numerous cases, with complete resolution of the hematoma and no long-term possible complications associated with the implantation of the stents (restenosis, thrombosis, etc.) Another option that has been suggested for the recovery of distal flow is the fenestration of the hematoma using a cutting or a scoring balloon.2 Fenestration allows the decompression of the hematoma, which improves flow in the compressed true lumen, thereby reducing the risk of hematoma progression.
In response to the question “how would I approach it?”, here follows my strategy for this case: in the first procedure I would not have used a IIb/IIIa inhibitor; I would have only used it in case of complex catheterization or increasing thrombus load. There is little experience on its safety profile in combination with ticagrelor or prasugrel, and the clinical practice guidelines only give a class IIb level of evidence C in patients with acute coronary syndrome treated with percutaneous coronary intervention who did not receive a P2Y12 inhibitor as a second antiplatelet agent. In the second procedure, I would perform an IVUS to assess the mechanism underlying vessel occlusion and when diagnosing the intramural hematoma I would also determine its distal spreading. If one healthy region were identified distally using the IVUS, I would implant one stent whose diameter would be adjusted to the size of the distal vessel in the healthy region and I would deploy it at nominal pressure until it overlaps with the former stent in an attempt to somehow contain the hematoma and avoid distal progression. If more than just one stent is needed, then I would first deploy the most distal stent making sure it lands on a healthy region. If, on the contrary, the IVUS shows diffuse damage towards the small caliber distal branches, in my attempt to recover the distal flow, I would first try balloon dilatation. In this case, damage to the posterior/posterolateral descending bifurcation is reported, which is why I would try to advance the guidewire towards both branches and also balloon dilatation. If flow is not recovered, then I would try fenestration with a slightly undersized cutting balloon. If normal coronary flow still fails to recover with all these measures, I would never implant the stent. If I couldn’t recover the distal flow, I would implant the stents following the strategy used by the authors in the case at stake, with long undersized stents while trying not to cause greater damage to the vascular wall.
Then I would schedule a new coronary angiography between 1 and 3 months later and I would assess, through one OCT, the presence of hematoma and the apposition of the stents. Since undersized stents have been used intentionally, we can anticipate finding no apposition, but even after achieving complete apposition following the implantation, the reabsorption of the hematoma can lead to stent malapposition. Also, the OCT would provide information on the early neointimal coverage that, on many occasions, can even cover the nonapposed stent struts and spontaneously solve the lack of apposition. In case of severe stent malapposition, especially if it had to do with a lack of strut coverage, I would consider stent post-dilatation3 to facilitate neointimal coverage and potentially reduce the risk of thrombosis.
REFERENCES
1. Saw J, Mancini GBJ, Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol. 2016;68:297-312.
2. Motreff P, Ronchard T, Sanguineti F, et al. Coronary Artery Fenestration:A Promising Technique for Rescue Management of Spontaneous Intramural Hematoma With Luminal Compression. JACC Cardiovasc Interv. 2018;11:1905-1907.
3. Sanchez-Recalde A, Moreno R, Jimenez-Valero S. Stenting of spontaneous intramural coronary haematoma:long-term consequences. Eur Heart J. 2008;29:1593.
E-mail address: sjvcardio@yahoo.es (S. Jiménez Valero).











