ABSTRACT
A substantial number of patients undergoing coronary angiography for angina or ischemia in noninvasive tests have coronary arteries without lesions or with nonsignificant stenosis. Many of these patients have nonobstructive myocardial ischemia (INOCA/ANOCA), which is an entity with prognostic importance that significantly affects patients’ quality of life. The absence of a proper diagnosis leads to inappropriate medical treatment, repeat diagnostic tests, and greater use of social and health resources. An adequate diagnostic strategy is required for individualized treatment that improves symptoms and quality of life. In this document from the SEC-Clinical Cardiology Association, SEC Interventional Cardiology Association, SEC-Ischemic Heart Disease and Acute Cardiac Care Association, and SEC-Cardiovascular Imaging Association of the Spanish Society of Cardiology, we provide simple and practical algorithms, with the aim of facilitating the early diagnosis and most appropriate treatment for patients with ANOCA.
Keywords: ANOCA. INOCA. Microvascular dysfunction. Vasospastic angina.
RESUMEN
Un número importante de aquellos pacientes en quienes se realiza coronariografía por angina o isquemia presentan en pruebas no invasivas arterias coronarias sin lesiones o con estenosis no significativas. Muchos de estos pacientes tienen isquemia miocárdica de causa no obstructiva (INOCA/ANOCA), una condición con importancia pronóstica que afecta de manera considerable la calidad de vida. La ausencia de un diagnóstico que haga posible un tratamiento médico efectivo acarrea la repetición de pruebas diagnósticas y un mayor uso de recursos sociosanitarios. Es necesaria una estrategia diagnóstica adecuada para poder realizar un tratamiento personalizado, que mejore los síntomas y la calidad de vida. En este documento de la SEC-Asociación de Cardiología Clínica, SEC Asociación de Cardiología Intervencionista, SEC-Asociación de Cardiopatía Isquémica y Cuidados Agudos Cardiovasculares, y SEC-Asociación de Imagen Cardiaca, se establecen unos algoritmos sencillos y prácticos con el objetivo de facilitar el diagnóstico precoz y el tratamiento más adecuado de los pacientes con ANOCA.
Palabras clave: ANOCA. INOCA. Disfunción microvascular. Angina vasoespástica.
Abbreviations ANOCA: angina with nonobstructive coronary arteries. CFR: coronary flow reserve. IMR: index of microcirculatory resistance. INOCA: ischemia with nonobstructive coronary artery disease. PET: positron emission tomography. SEC: Sociedad Española de Cardiología.
INTRODUCTION
Angina pectoris affects more than 100 million persons worldwide.1-5 According to the OFRECE study, the prevalence of angina in Spain is around 2.6%, indicating that there are more than 270 000 affected individuals.4 A significant number of stable patients referred for coronary angiography due to angina or a positive ischemia test do not have obstructive coronary artery disease.1 Many of these patients have ANOCA (angina with nonobstructive coronary arteries), or INOCA (ischemia with nonobstructive coronary artery disease) of nonobstructive origin. These 2 entities are manifestations of the same disease, which is why the recommendations provided by this document are applicable to both.
Angina pectoris is more prevalent among women (50%-70%) than men (30%-50%), although its true prevalence remains unknown.1-5 In these patients, angina or ischemia is produced by coronary vascular dysfunction due to vasomotor disorders of the epicardial vessels or arterioles, and/or coronary microvascular dysfunction.6-8
An important point is that, currently, angina pectoris is significantly underdiagnosed, and consequently many patients suffer its consequences without receiving potentially effective treatment. The reasons for this lack of diagnosis and treatment are various. First, there is the inertia associated with the paradigm that has dominated the diagnostic approach to patients with angina for decades focused on identifying coronary artery stenosis rather than vasomotor or coronary microvascular disorders. Additionally, patients with angina without coronary artery stenosis have generally been considered low-risk patients with poor response to conventional antianginal medical therapy.9 Second, and partly related to the previous point, many noninvasive techniques are based on identifying the regional ischemia that is characteristic of coronary artery stenosis (dysregulated contraction or isotope uptake during exertion or stress), making them less sensitive and specific for the detection of nonobstructive ischemia. Third, most cardiologists have not had access to the invasive techniques that provide objective evidence of vascular dysfunction in their patients. These intracoronary techniques have been considered the sole domain of interventional cardiologists, who do not usually play a key role in the management and follow-up of patients with INOCA. These barriers prevent the valuable information provided by invasive techniques from being used in the clinical management of these patients. Finally, patients with ANOCA/INOCA often have extracardiac diseases and conditions that require a multidisciplinary approach, complicating follow-up for specialized cardiologists.
In 2019, the European Society of Cardiology guidelines on the diagnosis and management of patients with chronic coronary syndrome represented a significant advance in the recognition of microvascular angina and the value of specific diagnostic techniques. Therefore, in the diagnostic approach in patients with suspected coronary microvascular angina, the guidelines indicate that coronary flow reserve (CFR) and microcirculatory resistance should be measured through pressure-guided techniques in patients with persistent symptoms but angiographically normal coronary arteries, or moderate stenosis and a normal fractional flow reserve (recommendation IIaB). Even the remaining recommendations, such as the administration of intracoronary acetylcholine during coronary angiography, or the use of transthoracic Doppler echocardiography of the anterior descending artery, cardiac magnetic resonance (CMR), or positron emission tomography (PET) for the noninvasive evaluation of CFR, have a lower level of recommendation (IIbB). In patients with suspected vasospastic angina, the guidelines recommend intracoronary provocation testing to identify coronary artery spasm (recommendation IIaB).10
However, over the past few years, numerous studies have been conducted in patients with ANOCA to assess the efficacy profile of new invasive diagnostic tests for their specific diagnosis, as well as randomized clinical trials assessing symptomatic improvement with individualized therapies. These trials consistently suggest that individualized and multidisciplinary approaches to these patients help to relieve symptoms, reduce the number of medical visits and prescribed therapies, and lower the costs associated with this syndrome.11-13
OBJECTIVES OF THIS DOCUMENT
This document is endorsed by the Clinical Cardiology Association, and the Interventional Cardiology Association, Ischemic Heart Disease and Acute Cardiac Care Association, and Cardiovascular Imaging Association of the Spanish Society of Cardiology (SEC) and aims to:
Review the various causes of ANOCA syndrome and current methods for its diagnosis and individualized treatment.
Propose a diagnostic and treatment algorithm for the approach to these patients in compliance with the clinical practice guidelines of the European Society of Cardiology on the management chronic coronary syndrome and the latest evidence.
Encourage various health care entities to create multidisciplinary pathways for the diagnosis, treatment, and targeted follow-up of these patients.
This document was drafted based on the interpretation of the latest scientific evidence, with an eminently practical focus so that the recommendations can be effectively applied in our setting. Each Association of the SEC provided scientific evidence and their view of their respective fields. Afterward, through consensus, they all created a single document including practical recommendations. The selection of the members that would eventually draft the document was left to the presidents of these Associations and was based on their clinical experience and expertise in the field.
IMPORTANCE OF ANOCA IN ROUTINE CLINICAL PRACTICE
While it has been acknowledged for decades that angina without coronary artery lesions could constitute a separate nosological entity (initially called syndrome X), routine clinical practice has paid little attention to affected patients, primarily due to the widespread notion that their prognosis is good.14 However, numerous subsequent studies in which the diagnosis of ANOCA was based on objective evidence of coronary vascular dysfunction, unlike that of syndrome X, consistently showed that nonobstructive ischemia has a significant prognostic impact. The risk of adverse coronary events in these patients is largely determined by factors such as plaque burden, demonstration of myocardial ischemia, microvascular dysfunction, and the presence of vasospasm or coronary endothelial dysfunction. For example, a study of 917 women with signs or symptoms of myocardial ischemia showed that the composite endpoint of myocardial infarction or cardiac death occurred in 6.5% of women without coronary artery disease, 12.8% of those with nonobstructive atherosclerosis, and 25.9% of those with obstructive coronary artery disease at 10 years of follow-up (figure 1).15 A meta-analysis of 54 studies and 35 039 patients confirmed an increased risk of nonfatal myocardial infarction and death, with an incidence rate of 0.98 per 100 person-years in patients with ANOCA at 5 years of follow-up. The risk was higher in individuals with confirmed ischemia (vs those without ischemia) and patients with nonobstructive coronary artery disease (vs those with normal coronary arteries).16
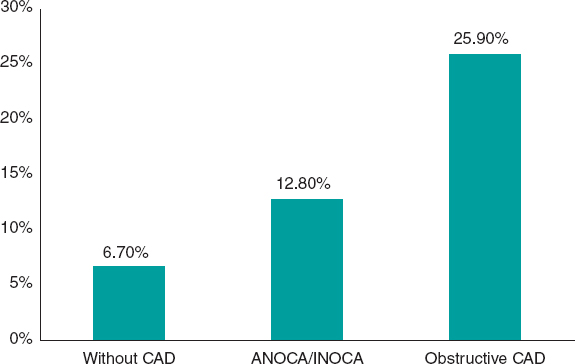
Figure 1. Risk of myocardial infarction or cardiovascular death at 10 years of follow-up in a cohort of women.15 ANOCA/INOCA, angina/ischemia with nonobstructive coronary arteries; CAD, coronary artery disease.
Similarly, even in the presence of angiographically normal coronary arteries, microvascular dysfunction demonstrated by a reduced CFR has proven to be a powerful determinant of the risk of death and myocardial infarction in these patients.17 Additionally, more cardiovascular complications, including stroke and heart failure,18 have also been reported in these individuals, along with a higher prevalence of small vessel cerebral disease.19 In conclusion, patients with coronary microvascular dysfunction, identified by an impaired CFR, have a higher risk of major cardiovascular events.20
Intracoronary acetylcholine provocation testing also allows coronary risk stratification. An abnormal response to intracoronary acetylcholine indicates vasomotor disorders due to endothelial dysfunction or smooth muscle cell hyperreactivity. In addition to causing vasospastic angina, coronary vasomotor disorders are associated with a higher long-term risk of cardiovascular events in patients with angina, especially when associated with increased coronary microcirculation.13,21 Even moderate vasoconstrictor responses to acetylcholine can be predictive of a worse prognosis in this context.20
Additionally, patients with ANOCA often show persistent symptoms, partly due to the lack of an early diagnosis, thus leading to treatment delay. This is associated with a higher number of unnecessary diagnostic tests to rule out obstructive coronary artery disease, visits to the emergency room, hospital admissions, anxiety, impaired quality of life, episodes of sick leave, and higher direct and indirect health care costs.16,22,23
Diagnosing INOCA is essential to provide effective therapies to control angina symptoms. The CorMicA trial (Coronary microvascular angina) included 151 patients with ANOCA who underwent cardiac catheterization and invasive functional assessment (CFR determination, index of microcirculatory resistance, and fractional flow reserve) followed by acetylcholine vasoreactivity testing.11 The patients were randomized to reveal their specific endotype, which would guide treatment based on the results (intervention group), vs standard treatment, which would be administered blind to the test results (control group). Targeted therapy was individualized based on the endotypes documented in the invasive study (vasospastic angina: smoking cessation, long-acting calcium channel blockers, long-acting nitrates, and lifestyle changes; microvascular angina: beta-blockers, lifestyle changes, possible angiotensin-converting enzyme inhibitors and statins; noncardiac chest pain: withdrawal of antianginal treatment). Targeted therapy was significantly associated with an improved angina-related quality of life at 6 months (measured using the Seattle Angina Questionnaire), disease perception, and treatment satisfaction, although no differences were reported in the risk of major adverse cardiovascular events. More antianginal drugs were prescribed in the intervention group (87.8% vs 48.7%; P < .001). While these results are very interesting, it is important to note that this was a single study with a limited number of patients.
ENDOTYPES OF PATIENTS WITH ANOCA
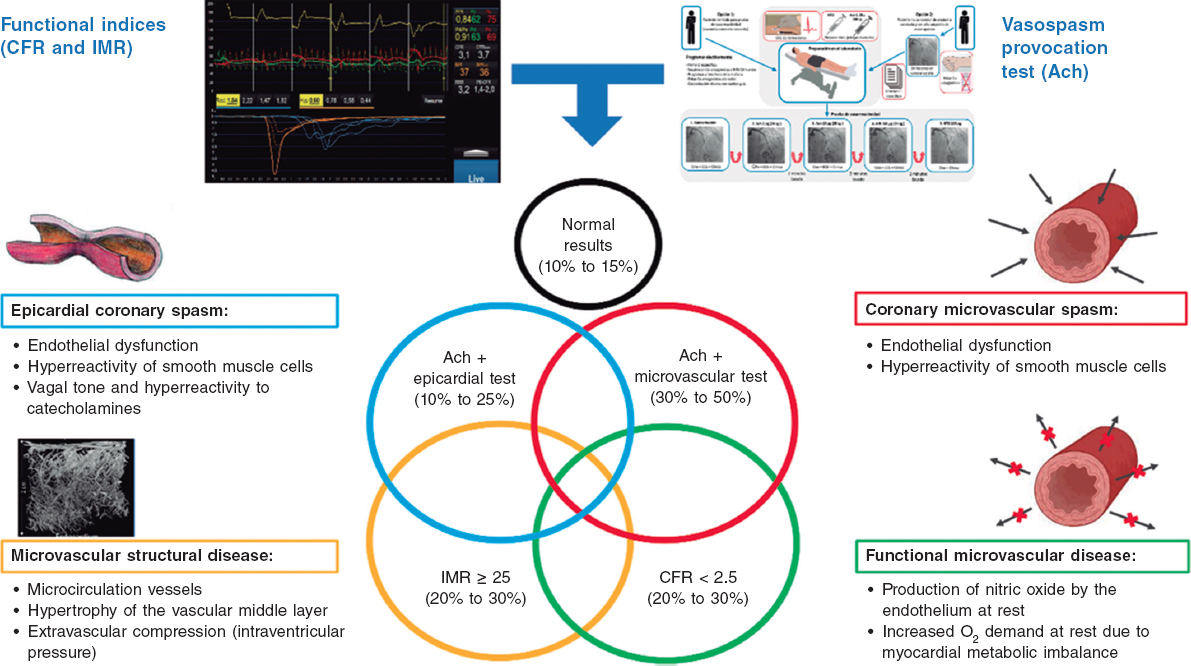
The specific causes of ANOCA are not yet fully described, and are likely multifactorial in most patients. Figure 2 illustrates the specific causes discovered so far and the pathophysiological mechanisms involved in their genesis. Of note, specific diagnostic techniques often do not allow us to differentiate among the various pathophysiological mechanisms. In fact, in many patients, these mechanisms overlap. Four pathophysiological mechanisms causing ANOCA have been described to date:
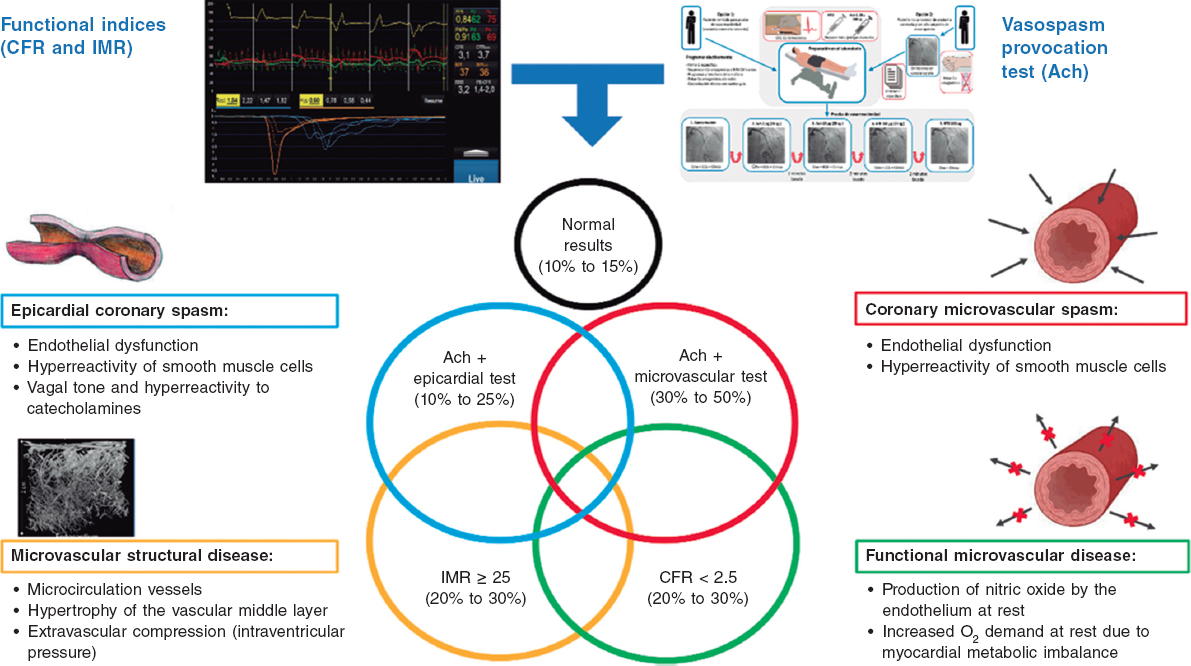
Figure 2. Possible results of an invasive functional study in a patient with ANOCA. Specific causes discovered to date with the pathophysiological mechanisms involved in their genesis. Ach, acetylcholine; ANOCA, angina with nonobstructive coronary arteries; CFR, coronary flow reserve; IMR, index of microcirculatory resistance. (Figure self-developed from Meeder et al.,1 Jansen et al.,3 Kunadian et al.,7 Kunadian et al.,34 and Hokimoto et al.35.)
Microvascular dysfunction due to structural changes to the microcirculation. The density of microvessels in patients with hypertensive cardiomyopathy is lower than that in patients without this condition.24 Remodeling of the coronary microcirculation has also been described, including arteriolar medial layer hypertrophy and induration in patients with hypertension, added to other cardiovascular risk factors, vascular infiltration by amyloid in cardiac amyloidosis, and reduced luminal caliber due to extrinsic compression in cases of ventricular hypertrophy or increased left intraventricular pressure.3,7,25 These changes reduce microcirculatory conductance, resulting in increased microvascular resistances (index of microcirculatory resistance [IMR] ≥ 25). Elevated IMR values are associated with older age and left ventricular hypertrophy, with no clear difference between the sexes.26,27
Functional microvascular disease. An increase in resting coronary blood flow, leading to reduced CFR levels has been reported, especially in women with few risk factors and no objectively observable structural heart disease.28 Although coronary flow is usually preserved at maximum hyperemia, many of these patients have a low exercise capacity. These patients may have an imbalance in oxygen availability (due to increased demand), with endothelial involvement being the main mechanism (due to increased nitric oxide synthesis).29 In addition, these patients tend to have a greater number of associated ischemic abnormalities in organs such as the kidneys, retina, and central nervous system, suggesting systemic involvement.30
Microvascular dysfunction due to microcirculatory spasm. Microvascular dysfunction due to vasospasm is more common in women with cardiovascular risk factors, with endothelial dysfunction likely playing a significant role. It is a common finding in larger and medium-sized arterioles and manifests as paradoxical vasoconstriction in response to increased myocardial oxygen demand, which becomes apparent after intracoronary of acetylcholine administration.3,7,19,31
Epicardial spasm. Epicardial spasm is not usually associated with traditional risk factors, except for smoking. This type of vasospasm is believed to be caused by 2 main mechanisms: endothelial dysfunction and smooth muscle cell hyperreactivity. These 2 mechanisms respond differently to stimuli from the autonomic nervous system, depending on whether the stimuli are from the sympathetic system (such as exercise or a cold stimulation test), or whether the stimuli are from the parasympathetic system and provoke an exacerbated response (eg, nocturnal spasms).19,32
CLINICAL CHARACTERISTICS OF PATIENTS WITH ANOCA
The first step in identifying patients with ANOCA is diagnostic suspicion. Patients with microvascular angina often report angina-like chest pain, typically on exertion, but it can also occur at rest. ANOCA is more common in women, and affected individuals generally show poor response to short-acting nitrates. In some cases, instead of angina, patients may have angina equivalents such as exertional dyspnea or atypical symptoms such as nausea, vomiting, dizziness, or fatigue. In microvascular spasm, which is also more common in women, unstable angina can occur with a variable response to nitrates.1-3
Regarding angina due to coronary vasomotor disorders, the spectrum and clinical signs of these disorders are much more varied than the pattern of Prinzmetal’s angina, which is a highly specific case of vasomotor disorder caused by an occlusive spasm of an epicardial vessel. However, this disorder is not representative of much more common substrates such as nonocclusive diffuse spasm and arteriolar or microvascular spasm. For example, in vasomotor disorders due to endothelial dysfunction, the dominant symptom is exertional angina, whereas in vasomotor disorders triggered by smooth muscle cell hyperreactivity of coronary vessels (such as in Prinzmetal’s angina), angina tends to occur at rest or becomes unstable, especially at night. Nevertheless, it can also be associated with exertional chest pain and be triggered by specific stimuli such as stress, cold, or an increase in vasoconstrictor humoral factors. Angina can also be associated with other conditions such as migraines or Raynaud’s phenomenon. Some anticancer drugs, such as 5-fluorouracil and capecitabine, among others, are known to be associated with vasospastic angina.33 Similarly, the initial clinical manifestation of epicardial spasm can be myocardial infarction with nonobstructive coronary arteries (MINOCA).19 This condition is often associated with smoking, unlike other traditional risk factors such as hypertension, diabetes mellitus, and dyslipidemia.19,32
NONINVASIVE DIAGNOSTIC APPROACH IN PATIENTS WITH ANOCA
The diagnostic approach to patients with ANOCA falls within the diagnostic process of chronic coronary syndrome as recommended by the current clinical practice guidelines and is initially noninvasive.10 However, it is important to note that the available scientific evidence—sometimes scarce—has already been analyzed, and consequently some statements are based not only on clinical trials but also on consensus among the authors of the document.
After angina is suspected, the patient should be referred to the cardiology unit for basic symptom examination, including an electrocardiogram, echocardiogram, a complete blood count, and clinical response to initial antianginal treatment. A noninvasive strategy is advised for most patients with nonlimiting symptoms and a low or intermediate pretest risk of obstructive coronary artery. This strategy involves noninvasive imaging modalities, including functional studies, based on surrogates of myocardial blood flow and CFR, and/or anatomical studies, mainly coronary computed tomography.3 The diagnostic tests performed will depend, among other factors, on the patient’s exercise tolerance and the availability and experience of each center (figure 3).1,3,7,34,35
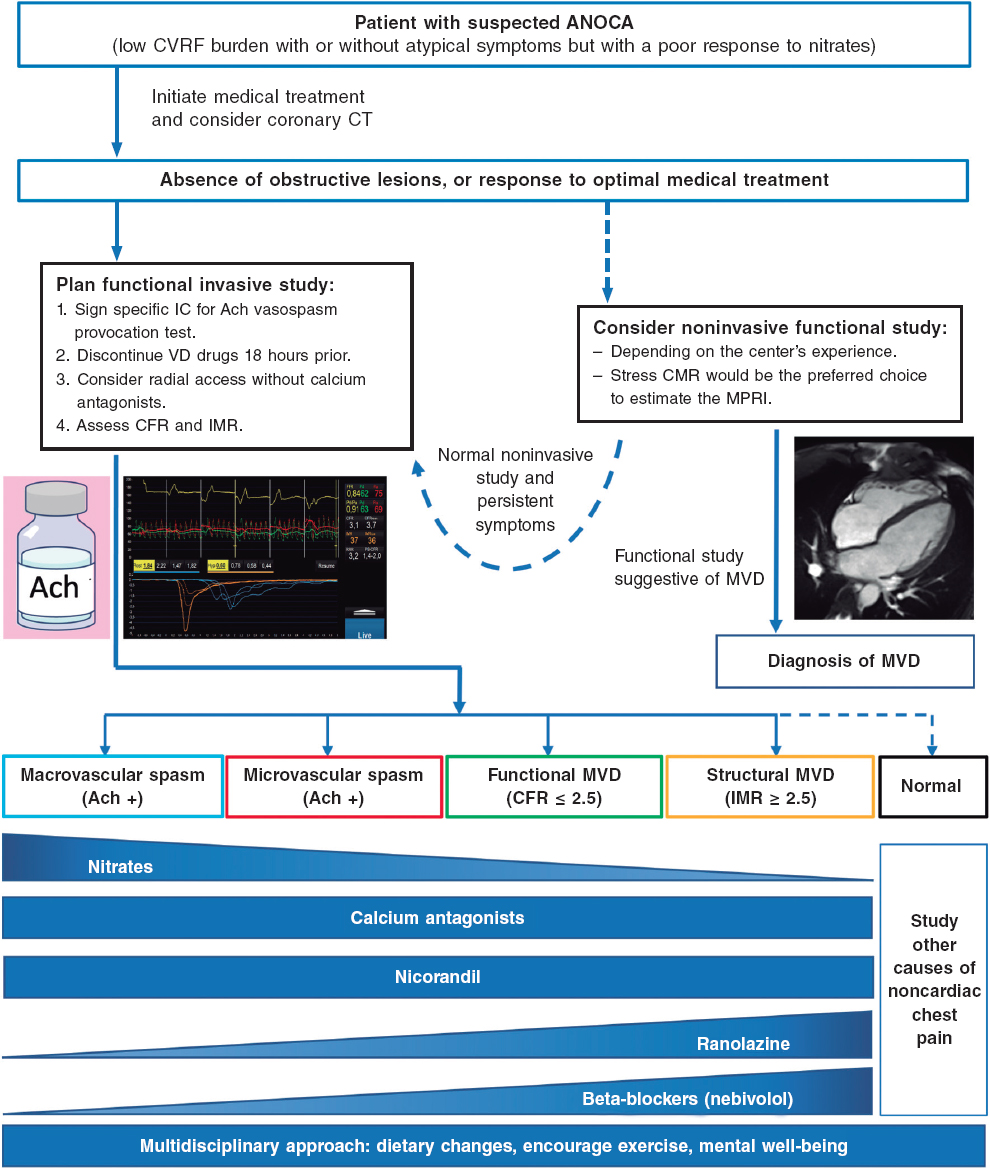
Figure 3. Diagnostic approach to patients with suspected ANOCA or INOCA. Ach, acetylcholine; ANOCA, angina with nonobstructive coronary arteries; IC, informed consent; CFR, coronary flow reserve; CT, computed tomography; CVRF, cardiovascular risk factors; IMR, index of microcirculatory resistance; INOCA, ischemia with nonobstructive coronary arteries; MPRI, myocardial perfusion reserve index; MRI, magnetic resonance imaging; MVD, microvascular dysfunction; VD, vasodilator. (Figure self-developed from Meeder et al.,1 Perera et al.,2 Jansen et al.,3 Kunadian et al.,7 Ang and Berry,31 Kunadian et al.,34 and Hokimoto et al.35.)
Of note, in many patients with ANOCA, noninvasive imaging modalities for detecting ischemia have low sensitivity for the diagnosis of most endotypes, especially those associated with coronary vasomotor disorders. In a registry of patients studied with noninvasive ischemia detection tests and invasive functional tests (considered the reference standard for diagnosis), only 50% of those with a low CFR showed abnormalities in the noninvasive imaging tests.36 In fact, no noninvasive stress test can reliably detect the presence of microvascular spasms or coronary endothelial dysfunction and a negative stress test does not exclude the presence of vasomotor coronary dysfunction, especially in symptomatic patients.7 The reasons for the low sensitivity of these techniques are diverse. However, an important reason is that they rely on visualizing regional differences among myocardial segments (nonuniform tracer uptake in single-photon emission computed tomography, differences in myocardial segment mobility in stress echocardiography). Given the characteristics of microvascular angina, in which ischemia can be widespread, it is difficult to find regional defects in noninvasive tests. Moreover, patients with vasospasms usually test negative in stress tests based on comparison between rest and hyperemia. Therefore, it is important to note that ANOCA should always be suspected in patients with suggestive chest pain and a normal coronary computed tomography scan, or without obstructive coronary artery disease (< 50% reduction in diameter), and in patients who test negative on noninvasive imaging modalities for ischemia detection. Currently, no imaging modality allows the direct anatomical visualization of coronary microcirculation in vivo in humans, which is why its evaluation relies on measuring parameters that reflect functional status, such as myocardial blood flow and myocardial flow reserve.7
However, certain ANOCA endotypes with low CFR and a high suspicion of microvascular angina can be diagnosed noninvasively through various imaging modalities such as PET, transthoracic Doppler echocardiography, contrast-enhanced transthoracic echocardiography, and CMR. CFR is defined as an increased flow between the resting state and maximum hyperemia. CFR values < 2 to 2.5 are considered pathological.1
PET allows determination of myocardial blood flow at rest and during hyperemia in absolute terms, which facilitates the calculation of CFR. Although PET is considered the reference noninvasive imaging modality and correlates well with invasive study (CFR < 2 is associated with a worse prognosis regardless of the severity of coronary artery disease),37 its availability is highly limited in our setting,3,38 due to its high cost and the need for specific cyclotron-produced radiation-emitting radiotracers, such as oxygen-15-labeled water, nitrogen-13-labeled ammonia, or rubidium-82, a potassium analog.
Transthoracic Doppler echocardiography allows for the measurement of baseline and hyperemic blood flow velocity (after adenosine administration) using pulsed-wave Doppler. CFR < 2.5 is considered diagnostic of microvascular dysfunction. However, this imaging modality requires highly trained personnel and can only be used in the left anterior descending coronary artery.3,39 On the other hand, contrast-enhanced transthoracic echocardiography using microbubbles allows estimation of myocardial perfusion flow based on its degree of opacification. The latter imaging modality has shown good correlation with PET, although there may be significant interobserver variability, thus requiring further validation in studies.40
Finally, CMR can determine myocardial perfusion using stress and contrast agents (gadolinium) to calculate the myocardial perfusion reserve index, which is a surrogate parameter of CFR. This imaging modality is more widely available than PET, and has less interobserver variability than echocardiographic studies, making it the most suitable imaging modality for the study microvascular dysfunction in our setting. However, CMR is still pending validation in the remaining ANOCA endotypes.3,41 Hyperemia or coronary vasodilation can be achieved through adenosine infusion, or the administration of a single bolus of regadenoson, and stress vs resting perfusion can be compared quantitatively. The diagnostic ability of stress CMR in microvascular dysfunction was demonstrated 2 decades ago.42 A myocardial perfusion reserve index < 1.84 has shown sensitivity and specificity rates of 73% and 74%, respectively, to predict abnormalities in invasive coronary physiology studies, with an area under the ROC curve of 0.78.41 A quantitative assessment of stress perfusion studies showed an even stronger correlation with invasive studies in a series of 65 patients (50 with stable angina, 46% of whom had no coronary artery lesions, and 15 healthy volunteers) to distinguish multivessel disease from microvascular dysfunction, with an area under the ROC curve of 0.94 (P < .001) for the absolute quantification of myocardial flow during stress < 1.82 mL/g/min.43 In this study, myocardial flow during stress correlated better with invasive measurements than with myocardial flow reserve. Additionally, its prognostic capability has also been demonstrated. In a series of 218 patients with angina and coronary arteries without epicardial lesions,44, a myocardial perfusion reserve index ≤ 1.47 was associated with a 3-fold higher risk of major cardiovascular events compared with patients with values > 1.47 (hazard ratio, 3.14; 95% confidence interval, 1.58-6.25; P = .001). In another series of 395 patients, myocardial perfusion reserve improved the prognostic value vs the baseline model (age, sex, and late enhancement) of the primary endpoint defined as a composite of cardiac death, nonfatal myocardial infarction, aborted sudden death, or late revascularization, at 460 days of follow-up. Moreover, this study confirmed that quantitative perfusion (defined as > 10% ischemic myocardium) was superior to qualitative perfusion (defined as perfusion defects in > 2 segments) in the assessment of ischemia.45 Rahman et al.46 also demonstrated that high-resolution CMR techniques using fully quantitative perfusion were properly accurate and outperformed visual assessment in detecting microvascular dysfunction.
Unfortunately, some of the tests that could help in the noninvasive functional diagnosis of patients with ANOCA/INOCA are not available in routine clinical practice in many centers in Spain, thus limiting the diagnostic approach in these patients.
Table 1 shows the diagnostic criteria for ANOCA, while figure 3 illustrates the complete diagnostic algorithm proposed for patients with ANOCA, specifying the initial strategy, when to schedule invasive studies, and the possible therapies based on the specific endotype.
Table 1. Diagnostic criteria for ANOCA
| Endotype | Physiopathology | Criteria | Comments |
|---|---|---|---|
| Microvascular angina | Coronary microvascular dysfunction | Myocardial ischemia symptoms | • Exertional or resting angina • Angina equivalent (exertional dyspnea) |
| Evidence of myocardial ischemia | • Positive ischemia detection test | ||
| Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography | ||
| Impaired coronary microvascular function | • Adenosine test: CFR ≤ 2.0 (2.5 according to the method), IMR ≥ 25, HMR ≥ 1.9 • Microvascular spasm (spontaneous or acetylcholine test): angina, EKG changes, without epicardial spasm (lumen reduction < 90%) | ||
| Vasospastic angina | Epicardial spasm | Symptoms | • Angina, more at rest, especially nocturnal • Reduced exercise tolerance, especially in the morning • Response to nitrates and calcium antagonists |
| EKG changes | • ST-segment changes (elevation/depression) ≥ 1 mV • New negative U waves | ||
| Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography | ||
| Coronary spasm | • Vasoconstriction > 90% with angina and spontaneous EKG changes, or after provocation test (acetylcholine) | ||
| Preserved coronary microvascular function | • Adenosine test: CFR > 2.0 (2.5 according to the method), IMR < 25, HMR < 1.9 | ||
| Mixed | Microvascular angina and epicardial spasm | Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
| Microvascular angina | • Microvascular dysfunction • Adenosine test: CFR ≤ 2.0 (2.5 according to the method); IMR ≥ 25, HMR ≥ 1.9 | ||
| Coronary spasm | • Angina + EKG changes + epicardial vasoconstriction (> 90%) | ||
| Noncardiac chest Pain | None | Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
| Normal functional tests | • Adenosine test: CFR > 2.0 (2.5 according to the method), IMR < 25, HMR < 1.9 • Negative acetylcholine test | ||
ANOCA, angina with nonobstructive coronary arteries; CFR, coronary flow reserve; CT, coronary computed tomography; EKG, electrocardiogram; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; IMR, index of microvascular resistance. Table based on data from Meeder et al.,1 Perera et al.,2 Jansen et al.,3 Kunadian et al.,7 Mejia-Renteria et al.,19 Ong et al.,25 Ang and Berry,31 Kunadian et al.,34 and Hokimoto et al.35. | |||
INVASIVE DIAGNOSTIC APPROACH IN PATIENTS WITH ANOCA
Although these are very safe procedures, there are risks involved in the invasive assessment of patients with suspected ANOCA. Therefore, it is of paramount importance that the health professionals involved should have specific training in performing and interpreting various tests. Adequate pathways should also be implemented. Currently, the use of 2 functional tests is advised, consisting of a vasospasm provocation test with intracoronary acetylcholine infusion and a microvascular function test using a pressure-temperature sensor-tipped wire at rest and during maximum pharmacological hyperemia.7,11,34,35
Vasospasm provocation testing with intracoronary acetylcholine is advised. Since the technical data sheet of acetylcholine does not include its intracoronary use, the pharmacy department of the medical center must be contacted for prior authorization. In most cases, patients must provide their prior written informed consent for the off-label use of the drug.47 This test has demonstrated high sensitivity and specificity rates (around 90% and 100%, respectively, depending on the patient’s characteristics) for diagnosing micro- and macrovascular vasospastic angina, with very few complications.47,48 Before the test is conducted, the use of long-acting vasodilator drugs should be avoided. A minimum of 18 hours without oral or topical vasodilator agents is advised to avoid false negatives. Although the use of beta-blockers may increase vasoconstriction after acetylcholine infusion, their discontinuation before the test is not advised if these drugs are deemed necessary. In procedures performed via the radial route, the use of calcium antagonists should also be avoided.47 Essentially, the test involves the infusion of increasing acetylcholine doses while simultaneously assessing the reproduction of the patient’s symptoms, changes in the 12-lead electrocardiogram, and the presence of spasms in the epicardial arteries > 90% of their baseline diameter. The Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology recently published a technical document on the performance and interpretation of this test.47
Microvascular function can be assessed using intracoronary Doppler, or pressure-temperature sensor-tipped wires. However, the only currently available guidewires are pressure-temperature sensor-tipped wires (Pressurewire X, Abbott, United States), which use the thermodilution method. Coronary thermodilution allows coronary flow values to be obtained at rest and during maximum hyperemia after the infusion of any microcirculation vasodilator agent (usually adenosine or its derivatives). These values are obtained after the infusion of 3 mL of physiological saline solution through the guide catheter and by measuring the transit time of this solution between the proximal segment of the artery and the distal segment, where the distal guidewire thermistor is located, both at rest and during maximum hyperemia. By obtaining flow data at rest and during maximum hyperemia, the CFR can be calculated, which under normal conditions should be > 2.5. CFR values ≤ 2.5 are considered diagnostic of microvascular dysfunction. Since the pressure of microcirculation perfusion (measured in the distal segment of the artery where the guidewire is located) can be obtained while performing the test during maximum hyperemia, the minimum microcirculation resistance (IMR) can be estimated. In studies performed in healthy patients, a cutoff value of 25 has been established. IMR values ≥ 25 are also indicative of microvascular dysfunction.7,34,35
There is another promising method in the invasive diagnosis of patients with ANOCA. Using the same pressure guidewire and a dedicated microcatheter (RayFlow, Hexacath, France), absolute coronary flow values (in mL/min) and absolute microcirculation resistances (in Wood units) can be obtained.49 Since these are absolute values, they partly depend on the perfusion territory of the artery and the studied segment. Currently, research is underway to develop an indexed approach using this method.50
THERAPEUTIC APPROACH IN PATIENTS WITH ANOCA
General approach
In patients with ANOCA, treatment should focus on relieving symptoms and improving the risk profile, quality of life, and prognosis. In this regard, early diagnosis, identification of the pathophysiological mechanisms involved, and early initiation of treatment tailored to the INOCA endotype are key to achieving therapeutic success.1,3,7,25,31,34,35,51-54 However, currently available studies of specific medical treatment for this condition are small, with heterogeneous methodologies and variable results, which makes it difficult to establish robust recommendations for the therapeutic management of these patients.
Lifestyle changes and control of cardiovascular risk factors
First, given the impact of cardiovascular risk factors on the development of coronary microvascular dysfunction and epicardial spasm, effective control of these risk factors is essential, including lifestyle changes (weight loss, physical exercise, smoking cessation, stress reduction), and appropriate pharmacological therapies.10 To reduce the risk of coronary vasospasm, it is important to avoid triggering factors such as smoking and the use of certain drugs (cocaine and amphetamine).10
Statins are beneficial not only due to their effect on lipid profile, but also due to their positive effect on endothelial function and in preventing the development of coronary spasms.55,56 Renin-angiotensin-aldosterone system inhibitors are beneficial to reduce blood pressure and improve endothelial function. In fact, these drugs have been reported to have positive effects on both coronary microvascular dysfunction and epicardial coronary vasospasm.55-57 The role of aspirin in patients without known cardiovascular disease is controversial.55,56 In the Japanese guidelines, aspirin is not advised in the absence of angiographically confirmed stenosis in patients with vasospasm (class IIIB indication).35
Antianginal treatment
Antianginal treatment is crucial for symptom relief. Preferential use of drugs that reduce myocardial oxygen consumption is advised in patients with a structural endotype of INOCA (microvascular dysfunction), such as beta-blockers or calcium channel blockers (ivabradine may also be considered in certain cases), along with other drugs such as ranolazine, trimetazidine, and nicorandil. On the other hand, calcium channel blockers, nitrates, nicorandil, or a combination of these, are advised in patients with a vasomotor endotype of INOCA (whether epicardial or microvascular spasm) (table 2).1,3,7,25,31,34,35,51-54
Table 2. Therapeutic approach for patients with ANOCA or INOCA
| General treatment | |||
|---|---|---|---|
| Lifestyle changes | • Mediterranean diet • Physical exercise • Weight control • Stress reduction | ||
| Cardiovascular risk factor control | • Hypertension • Dyslipidemia • Diabetes • Smoking cessation | ||
| Aspirin | • With previous CVD • Without previous CVD, its use is controversial | ||
| ACEI or ARA II | • Blood pressure reduction • Improvement in endothelial function: possible benefit in microvascular coronary dysfunction and coronary vasospasm | ||
| Statins | • Reduction in total cholesterol and LDL • Improvement in endothelial function • Possible benefit in vasospastic angina | ||
| Anti-anginal drugs | Microvascular angina | Beta-blockers | • Decreased myocardial oxygen consumption* |
| Calcium antagonists | • Decreased myocardial oxygen consumption • Vascular smooth muscle relaxation | ||
| Ranolazine | • Improvement in microvascular perfusion reserve | ||
| Trimetazidine | • Increased cellular tolerance to ischemia | ||
| Vasospastic angina | Calcium antagonists | • Decreased myocardial oxygen consumption • Decreased coronary spasm via relaxation of vascular smooth muscle | |
| Nitrates | • Decreased myocardial oxygen consumption • Decreased coronary spasm via relaxation of vascular smooth muscle | ||
| Nicorandil | • Coronary vasodilator effect | ||
| Microvascular angina + vasospastic angina | Calcium antagonists, nitrates, ranolazine, trimetazidine, nicorandil | ||
* Consider the use of nebivolol due to its antioxidant properties through nitric oxide. ACEI, angiotensin-converting enzyme inhibitors; ANOCA, angina with nonobstructive coronary arteries; ARA II, angiotensin II receptor antagonists; CVD, cardiovascular disease; INOCA, ischemia with nonobstructive coronary arteries; LDL, low-density lipoproteins. Table based on data from Meeder et al.,1 Jansen et al.,3 Kunadian et al.,7 Kobayashi et al.,26 Ang and Berry,31 Kunadian et al.,34 Hokimoto et al.,35 Beltrame et al.,51 Mehta et al.,52 Seitz et al.,53 and Abouelnour et al.54. | |||
There is some evidence on nebivolol compared with other beta-blockers, due to its potential vasodilatory effect that targets the production of nitric oxide.58 A beneficial effect of carvedilol has also been suggested by improving endothelium-dependent dilation.59 A randomized clinical trial of 81 patients demonstrated the benefit of ranolazine treatment in relieving symptoms in patients with CFR values < 2.5.60 Diltiazem treatment shows no benefits in improving symptoms, quality of life, or coronary microvascular function in the randomized EDIT-CMD trial of 73 patients with ANOCA in a 6-week course of treatment, although there was a reduction in induced epicardial vasospasms.12 Finally, there are promising potential benefits associated with drugs that have new therapeutic targets, such as cilostazol, a phosphodiesterase 3 inhibitor that targets coronary vasospasm,61 or zibotentan, a selective endothelin A antagonist with benefits on microcirculation and endothelial dysfunction,62 or fasudil, a rho-kinase enzyme inhibitor capable of reducing the IMR in patients with a positive vasospasm provocation test and elevated IMR.13
Treatment for resistant angina
The use of drugs such as low-dose tricyclic antidepressants (which modulate norepinephrine uptake and have anticholinergic effects, which can induce analgesia), or neurostimulators that block the transfer of pain at the spinal cord has been proposed in patients with resistant angina, and even coronary interventions in the case of vasospastic angina refractory to medical therapy.51
Patient follow-up
The follow-up of these patients should be coordinated between primary care physicians and cardiologists, and once symptoms are under control, follow-up should preferably be conducted in primary care units, with referrals to cardiology if there is decompensation. In addition, given the particularities of ANOCA, it is essential to inform patients about their disease and its implications. A multidisciplinary approach is necessary since other health professionals, such as psychologists, internists, and pain clinics, may sometimes be required.
Future lines of research
Finally, ongoing clinical trials are currently exploring whether intensive treatment of coronary atherosclerosis with high-intensity statins, renin-angiotensin-aldosterone system inhibitors, and low doses of aspirin improves angina and ischemia. The WARRIOR trial (NCT03417388) is studying whether such treatment improves outcomes, and the MINOCA-BAT trial (NCT03686696) is investigating whether the combined use of beta-blockers and renin-angiotensin-aldosterone system inhibitors reduces major cardiovascular clinical events.
CONCLUSIONS
Patients with suspected ANOCA exhibit a wide array of presentations that can currently be diagnosed and treated with effective individualized therapies. It is important for clinical cardiologists to become familiar with the various abnormalities in patients with ANOCA, and the currently available diagnostic and therapeutic tools. Invasive diagnostic tests constitute a new option requiring specific training for their correct performance and interpretation, as well as CMR with adenosine or regadenoson for myocardial perfusion calculation. In conclusion, specific actions need to be taken by all health centers to create diagnostic and therapeutic protocols for the management of these patients.
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence has not been used in the preparation of this document.
AUTHORS’ CONTRIBUTIONS
All authors contributed equally to the conception, literature search, development, drafting, reading, and final approval of the manuscript. C. Escobar served as the consensus coordinator.
CONFLICTS OF INTEREST
J. Escaned, a recipient of the Intensification of Research Activity Project INT22/00088 from Instituto de Salud Carlos III, declared speaker’s fees for his involvement in educational activities for Abbott and Philips. C. Escobar, J.M. Gámez, and V. Barrios declared lecture fees from Menarini. The remaining authors declared no conflicts of interest whatsoever.
REFERENCES
1. Meeder JG, Hartzema-Meijer MJ, Jansen TPJ, Konst RE, Damman P, Elias-Smale SE. Outpatient Management of Patients With Angina With No Obstructive Coronary Arteries:How to Come to a Proper Diagnosis and Therapy. Front Cardiovasc Med. 2021;8:716319.
2. Perera D, Berry C, Hoole SP, et al. Invasive coronary physiology in patients with angina and non-obstructive coronary artery disease:a consensus document from the coronary microvascular dysfunction workstream of the British Heart Foundation/National Institute for Health Research Partnership. Heart. 2022;109:88-95.
3. Jansen TPJ, Konst RE, Elias-Smale SE, et al. Assessing Microvascular Dysfunction in Angina With Unobstructed Coronary Arteries:JACC Review Topic of the Week. J Am Coll Cardiol. 2021;78:1471-1479.
4. Alonso JJ, Muñiz J, Gómez-Doblas JC, et al. Prevalencia de angina estable en España. Resultados del estudio OFRECE. Rev Esp Cardiol. 2015;68:691-699.
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291-1300.
6. Rahman H, Ryan M, Lumley M, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation. 2019;140:1805-1816.
7. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology and Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
8. Taqueti VR. Coronary microvascular dysfunction in vasospastic angina:provocative role for the microcirculation in macrovessel disease prognosis. J Am Coll Cardiol. 2019;74:2361-2364.
9. Luu JM, Wei J, Shufelt CL, et al. Clinical Practice Variations in the Management of Ischemia With No Obstructive Coronary Artery Disease. J Am Heart Assoc. 2022;11:e022573.
10. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
11. Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina:the CorMicA trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841-2855.
12. Jansen TPJ, Konst RE, de Vos A, et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA:The EDIT-CMD Randomized Clinical Trial. JACC Cardiovasc Imaging. 2022;15:1473-1484.
13. Suda A, Takahashi J, Hao K, et al. Coronary Functional Abnormalities in Patients With Angina and Nonobstructive Coronary Artery Disease. J Am Coll Cardiol. 2019;74:2350-2360.
14. Kaski JC, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA, Rosano GM. Cardiac syndrome X:clinical characteristics and left ven-tricular function:long-term follow-up study. J Am Coll Cardiol. 1995;25:807-814.
15. Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease:findings from the national heart, lung, and blood institute-sponsored women's ischemia syndrome evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134-141.
16. Radico F, Zimarino M, Fulgenzi F, et al. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease:a systematic review and meta-analysis. Eur Heart J. 2018;39:2135-2146.
17. Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia:results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825-2832.
18. Patel S, Fung M, Liang Z, Butalia S, Anderson TJ. Temporal Trends of the Prevalence of Angina With No Obstructive Coronary Artery Disease (ANOCA). Can J Cardiol. 2023;39:63-70.
19. Mejia-Renteria H, Travieso A, Matías-Guiu JA, et al. Coronary microvascular dysfunction is associated with impaired cognitive function:the Cerebral-Coronary Connection study (C3 study). Eur Heart J. 2023;44:113-125.
20. Boerhout CKM, de Waard GA, Lee JM, et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease;from the multicentre international ILIAS registry. EuroIntervention. 2022;18:719-728.
21. Grigorian-Shamagian L, Oteo JF, Gutiérrez-Barrios A, et al. Endothelial dysfunction in patients with angina and non-obstructed coronary arteries is associated with an increased risk of mayor cardiovascular events. Results of the Spanish ENDOCOR registry. Int J Cardiol. 2023;370:18-25.
22. Jespersen L, Abildstrøm SZ, Hvelplund A, Prescott E. Persistent angina:highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571-581.
23. Schumann CL, Mathew RC, Dean JL, et al. Functional and Economic Impact of INOCA and Influence of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021;14:1369-1379.
24. Nadruz W. Myocardial remodeling in hypertension. J Hum Hypertens. 2015;29:1-6.
25. Ong P, Camici PG, Beltrame JF, et al.;Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20.
26. Kobayashi Y, Fearon WF, Honda Y, et al. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients With Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1433-1441.
27. Chung JH, Lee KE, Lee JM, et al. Effect of Sex Difference of Coronary Microvascular Dysfunction on Long-Term Outcomes in Deferred Lesions. JACC Cardiovasc Interv. 2020;13:1669-1679.
28. Nardone M, McCarthy M, Ardern CI, et al. Concurrently Low Coronary Flow Reserve and Low Index of Microvascular Resistance Are Associated With Elevated Resting Coronary Flow in Patients With Chest Pain and Nonobstructive Coronary Arteries. Circ Cardiovasc Interv. 2022;15:e011323.
29. Rahman H, Demir OM, Khan F, et al. Physiological Stratification of Patients With Angina Due to Coronary Microvascular Dysfunction. J Am Coll Cardiol. 2020;75:2538-2549.
30. Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565-2568.
31. Ang DTY, Berry C. What an Interventionalist Needs to Know About INOCA. Interv Cardiol. 2021;16:e32.
32. Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774-1782.
33. Matsumoto T, Saito Y, Saito K, et al. Relation Between Cancer and Vasospastic Angina. Adv Ther. 2021;38:4344-4353.
34. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology &Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16:1049-1069.
35. Hokimoto S, Kaikita K, Yasuda S, et al. JCS/CVIT/JCC 2023 Guideline Focused Update on Diagnosis and Treatment of Vasospastic Angina (Coronary Spastic Angina) and Coronary Microvascular Dysfunction. Circ J. 2023;87:879-936.
36. Lee SH, Shin D, Lee JM, et al. Clinical Relevance of Ischemia with Nonobstructive Coronary Arteries According to Coronary Microvascular Dysfunction. J Am Heart Assoc. 2022;11:e025171.
37. Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58;740-748.
38. Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int J Cardiovasc Imaging. 2017;33:1021-1031.
39. Michelsen MM, Mygind ND, Pena A, et al. Transthoracic Doppler echocardiography compared with positron emission tomography for assessment of coronary microvascular dysfunction:the iPOWER study. Int J Cardiol. 2017;228:435-443.
40. Vogel R, Indermühle A, Reinhardt J, et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography:algorithm and validation. J Am Coll Cardiol. 2005;45:754-762.
41. Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute–sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8:e002481.
42. Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948-1953.
43. Kotecha T, Martinez-Naharro A, Boldrini M, et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction:Validation Against Invasive Coronary Physiology. JACC Cardiovasc Imaging. 2019;12:1958-1969.
44. Zhou W, Lee JCY, Leung ST, et al. Long-Term Prognosis of Patients With Coronary Microvascular Disease Using Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2021;14:602-611.
45. Sammut EC, Villa ADM, Di Giovine G, et al. Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2018;11:686-694.
46. Rahman H, Scannell CM, Demir OM, et al. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021;14:978-986.
47. Gutiérrez E, Gómez-Lara J, Escaned J, et al. Assessment of the endothelial function and spasm provocation test performed by intracoronary infusion of acetylcholine. Technical report from the ACI-SEC. REC Interv Cardiol. 2021;3:286-296.
48. Montone RA, Rinaldi R, Del Buono MG, et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and non-obstructive coronary arteries. EuroIntervention. 2022;18:e666-e676.
49. Rivero F, Gutiérrez-Barrios A, Gomez-Lara J, et al. Coronary microvascular dysfunction assessed by continuous intracoronary thermodilution:A comparative study with index of microvascular resistance. Int J Cardiol. 2021;333:1-7.
50. de Vos A, Jansen TPJ, van't Veer M, et al. Microvascular Resistance Reserve to Assess Microvascular Dysfunction in ANOCA Patients. JACC Cardiovasc Interv. 2023;16:470-481.
51. Beltrame JF, Tavella R, Jones D, Zeitz C. Management of ischaemia with non-obstructive coronary arteries (INOCA). BMJ. 2021;375:e060602.
52. Mehta PK, Huang J, Levit RD, Malas W, Waheed N, Bairey Merz CN. Ischemia and no obstructive coronary arteries (INOCA):A narrative review. Atherosclerosis. 2022;363:8-21.
53. Seitz A, Martínez Pereyra V, Sechtem U, Ong P. Update on coronary artery spasm 2022 –A narrative review. Int J Cardiol. 2022;359:1-6.
54. Abouelnour A, Gori T. Vasomotor Dysfunction in Patients with Ischemia and Non-Obstructive Coronary Artery Disease:Current Diagnostic and Therapeutic Strategies. Biomedicines. 2021;9:1774.
55. Ong P, Athanasiadis A, Sechtem U. Treatment of Angina Pectoris Associated with Coronary Microvascular Dysfunction. Cardiovasc Drugs Ther. 2016;30:351-356.
56. Picard F, Sayah N, Spagnoli V, Adjedj J, Varenne O. Vasospastic angina:A literature review of current evidence. Arch Cardiovasc Dis. 2019;112:44-55.
57. Choi BG, Jeon SY, Rha SW, et al. Impact of Renin-Angiotensin System Inhibitors on Long-Term Clinical Outcomes of Patients With Coronary Artery Spasm. J Am Heart Assoc. 2016;5:e003217.
58. Erdamar H, Sen N, Tavil Y, et al. The effect of nebivolol treatment on oxidative stress and antioxidant status in patients with cardiac syndrome-X. Coron Artery Dis. 2009;20:238-244.
59. Matsuda Y, Akita H, Terashima M, et al. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140:753-759.
60. Rambarat CA, Elgendy IY, Handberg EM, et al. Late sodium channel blockade improves angina and myocardial perfusion in patients with severe coronary microvascular dysfunction:Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction ancillary study. Int J Cardiol. 2019;276:8-13.
61. Shin ES, Lee JH, Yoo SY, et al. A randomised, multicentre, double blind, placebo controlled trial to evaluate the efficacy and safety of cilostazol in patients with vasospastic angina. Heart. 2014;100:1531-1536.
62. Morrow AJ, Ford TJ, Mangion K, et al. Rationale and design of the Medical Research Council's Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial. Am Heart J. 2020;229:70-80.
* Corresponding author.
E-mail address: escobar_cervantes_carlos@hotmail.com (C. Escobar).
@JEscaned; @AntoniCarolRuiz; @S_Raposeiras; @jmgamez3; @rfreixap; @Ana_Viana_T; @clinica_sec; @AgudosSEC
ABSTRACT
Coronary artery calcification is probably the main determinant of the poor outcome of percutaneous coronary interventions and is associated with higher rates of adverse events. There are currently different balloon or specific device-based plaque modification techniques available. Knowing their characteristics and proper use is key for the optimal treatment of calcified lesions. This position paper—promoted by the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC)—describes existing plaque modification techniques currently available and proposes an algorithm for the management of calcified lesions.
Keywords: Calcified coronary lesions. Plaque modification techniques. Intracoronary imaging modalities.
RESUMEN
La calcificación coronaria es probablemente el mayor determinante de un mal resultado de la angioplastia y se asocia a mayores tasas de eventos adversos. En la actualidad existen distintas técnicas de modificación de la placa basadas en balones o en dispositivos específicos. El conocimiento de sus características y su uso adecuado son aspectos clave para el tratamiento óptimo de las lesiones calcificadas. Este artículo de posicionamiento, promovido desde la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (ACI-SEC), describe las técnicas de modificación de la placa existentes en la actualidad y propone un algoritmo para el tratamiento de la lesión calcificada.
Palabras clave: Lesiones coronarias calcificadas. Técnicas de modificación de la placa. Imagen intracoronaria.
Abbreviations
CB: cutting balloon. ELCA: excimer laser coronary angioplasty. ICL: intracoronary lithotripsy. OA: orbital atherectomy. RA: rotational atherectomy. SB: scoring balloon.
IMPLICATIONS OF CALCIFICATION IN PERCUTANEOUS CORONARY INTERVENTIONS
Vascular calcification is a process closely associated with atherosclerosis. It can occur in the media (in peripheral arteries mainly) or intima layers (in coronary arteries). In the context of coronary atherosclerosis it debuts in intermediate or advanced stages in plaque evolution due to conversion of smooth muscle cells into osteoblastic phenotypes and infiltration of atheromatous plaque due to macrophages that clear out apoptotic smooth muscle cells and contain calcified vesicles.1 Atheromatous plaque calcification can take different shapes that probably correspond to different stages of the same disease like microcalcifications (< 15 μm), punctiform calcifications (circumferential arc < 90º), leaves or thin calcium layers (circumferential arc > 90º or > 3 mm in length), and calcium nodules.1
The main risk factors associated with coronary artery calcification are age, Caucasian race, diabetes mellitus, and chronic kidney disease.1
The prevalence of coronary artery calcification if variable based on the population studied and the diagnostic method used.2 The traditional angiographic definition of moderate calcification described radiopacities seen during cardiac motion while severe calcification is described as radiopacities seen without cardiac motion, usually on both sides of the arterial lumen. The prevalence of moderate or severe calcification is between 18% and 60%.3,4
Calcification complicates percutaneous coronary interventions (PCI) for various reasons: a) resistance to the advance of different devices especially in the presence of tortuosity (eventually, “non-crossable” lesions); b) reduced plaque compliance that will eventually require higher pressure in dilatation balloons or plaque modification devices (“non-dilatable” lesions); and c) difficulties advancing the stent and expanding it.5 Other issues would be malapposition and polymer damage that can lead to a non-homogeneous release of antiproliferative drugs. Everything combined makes calcification one of the major determinants of the SYNTAX score,6 and associated with worse PCI outcomes and higher rates of adverse events at follow-up including mortality in patients with extremely calcified coronary artery lesions.7 In addition, it increases the rate of procedural complications associated with calcification per se and with the tools necessary for treatment: coronary artery dissection, loss of side branches, PCI material entrapment, stent distortion or even stent loss, and the dreaded coronary artery perforation that is particularly severe since it is very difficult to advance any kind of sealing materials.8
To stop these issues and their prognostic implications from happening numerous plaque modification devices have been developed. The appropriate use of these devices is essential to perform safe and effective PCIs on calcified coronary artery lesions.
This position paper has been promoted by the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) with contributions from different expert professionals in this setting. It describes the plaque modification techniques currently available in our field and proposes an algorithm for the management of calcified coronary artery lesions.
INTRACORONARY IMAGING MODALITIES FOR CALCIFIED LESION ASSESSMENT
Intracoronary imaging modalities play a key role in the assessment of calcified coronary artery lesions. The use of optical coherence tomography (OCT) or intravascular ultrasound (IVUS) can be very useful to improve the detection and assesment of coronary artery calcium, select the plaque modification technique, and optimize results especially in association with stent expansion.
Calcification detection and assessment
Angiography is a limited sensitivity tool to detect coronary artery calcium. Unlike angiography both the IVUS and the OCT have higher sensitivity and specificity to assess the characteristics and degree of calcification, which are basic aspects to determine the therapeutic options.2,9 Table 1 shows the differences of these 2 intracoronary imaging modalities regarding calcium detection. The main difference between the 2 is that, since calcium creates posterior acoustic shadowing on the IVUS, calcium thickness cannot be properly assessed. As alternative marker, the presence of reverberations on the IVUS has been associated with the presence of thinner calcium layers (< 0.5 mm). On the OCT, parietal calcium does not create posterior acoustic shadowing and, therefore, its thickness can be assessed accurately. Nodular calcium, however, creates a shadow in both the IVUS and the OCT (figure 1).
Table 1. Intracoronary imaging modalities for calcified coronary artery lesion calcification
| Imaging modality | Sensitivity | Specificity | Calcium pattern | Calcium arc | Calcium length | Calcium thickness | Disadvantages |
|---|---|---|---|---|---|---|---|
| OCT | ++++ | ++++ | Parietal calcium: low reflectivity structure with demarcated borders and without posterior shadowing (figure 1A) Calcium nodule: Protruding structure into the lumen with posterior shadowing (figure 1C) | Allows quantification | Allows quantification | Can be measured | Requires clearing the blood from the vessel lumen for image acquisition. This can increase the contrast volume compared to IVUS Does not acquire proper images of ostial lesions |
| IVUS | +++++ | ++++ | Parietal calcium: hyperechogenic structure with posterior shadowing ( Calcium nodule: Structure protruding into the lumen with posterior shadowing ( | Allows quantification | Allows quantification | Cannot be measured due to posterior shadowing Reverberations are a marker of thin calcium (< 0.5 mm) | Posterior shadowing complicates calcium thickness assessment In the 20 MHz IVUS the limited resolution and near-field clutter artifact can complicate the definition of calcium depth with respect to lumen in severe lesions |
IVUS, intravascular ultrasound; OCT, optical coherence tomography. | |||||||
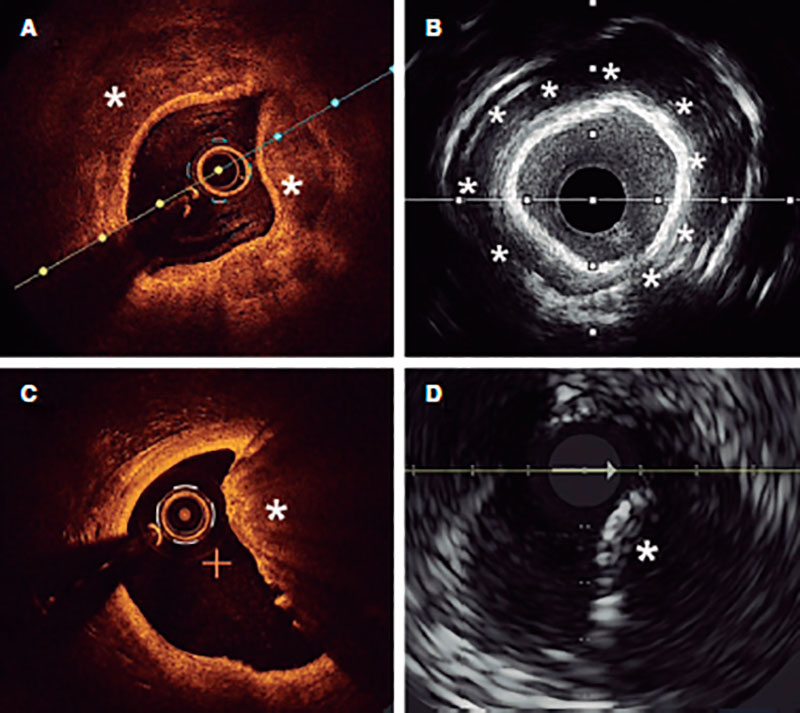
Figure 1. Coronary artery calcium assessment with IVUS and OCT. A: Parietal calcium on the OCT, low reflectivity structure with demarcated borders (asterisk). B: Parietal calcium on the IVUS, hyperechogenic structure with posterior shadowing. C: Calcium nodule on the OCT, structure protruding into the lumen with posterior shadowing. D: Calcium nodule on the IVUS, structure protruding into the lumen with posterior shadowing.
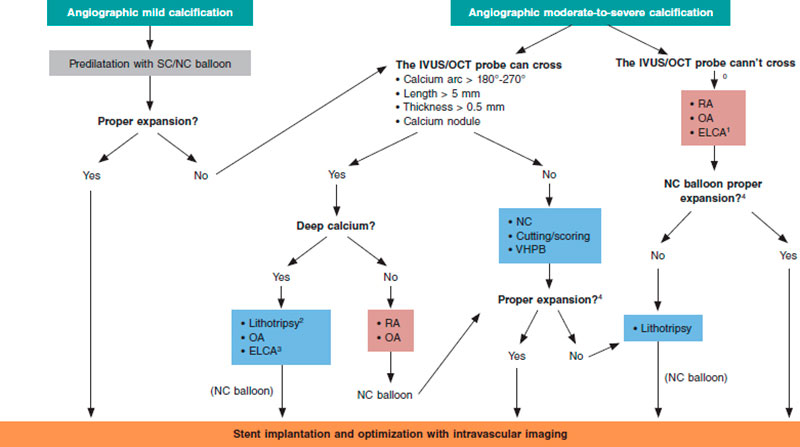
Different scoring systems have been developed for both intracoronary imaging modalities (table 2) including the characteristics of calcification that have been associated with stent underexpansion. The first OCT suitable scale ever developed includes 3 different parameters: calcium arc > 180º (score = 2), length > 5 mm (score = 1), and thickness > 0.5 mm (score = 1). Lesions with scores > 2 have a higher risk of stent underexpansion if proper plaque preparation is lacking.5 A similar scale has been developed for IVUS using 4 different criteria: calcium arc > 270º with > 5 mm in length (score = 1), calcium arc > 360º (score = 1), presence of calcified nodule (score = 1) and adjacent vessel < 3.5 mm (score = 1). Scores ≥ 2 are indicative of the need for plaque modification prior to stenting.10
Table 2. Intracoronary calcium scores based on optical coherence tomography and intravascular ultrasound
| OCT | IVUS | |||
|---|---|---|---|---|
| Scores | Scores | |||
| Máximo arco de calcio | ≤ 180° | 0 | ≤ 270º | 0 |
| > 180° (> 50%* of arc circumference) | 2 | 270º and > 5 mm in length | 1 | |
| 360º | 1 | |||
| Máximo grosor de calcio | ≤ 0.5 mm | 0 | ||
| > 0.5* mm | 1 | |||
| Longitud de calcio | ≤ 5 mm | 0 | ||
| > 5* mm | 1 | |||
| Type of calcium | Non-nodular | 0 | ||
| Nodule | 1 | |||
| Vessel diameter | ≥ 3.5 mm | 0 | ||
| < 3.5 mm | 1 | |||
IVUS, intravascular ultrasound; OCT, optical coherence tomography. | ||||
Selection of plaque modification techniques under intracoronary imaging modality guidance
The characteristics of calcium as seen on the intracoronary imaging modalities can contribute to the selection of the most adequate plaque modification technique. There is in depth information on this aspect in the last section of the document but, overall, lesions where calcium does not have underexpansion risk criteria can be treated with high-pressure or modified balloons (scoring, cutting). However, if these criteria exist it will be necessary to use more advanced plaque modification techniques. Added to these criteria, we should also mention calcium depth since some imaging modalities only act on the superficial—not deep—layer of the plaque.
Optimization of stenting under intracoronary imaging modality guidance
Both the IVUS and the OCT allow us to determine whether proper stent expansion has been achieved. This is especially relevant in calcified coronary artery lesions that happen to be the ones that are most associated with stent underexpansion, the parameter most strongly associated with stent failure.11 Proper apposition and lack of dissection or significant border hematoma, as well as proper lesion coverage are other optimization parameters under intracoronary imaging modality guidance that should also be assessed after stenting.12
BALLOON-FREE TECHNIQUES
Rotational atherectomy
The rotational atherectomy (RA) technique uses a metal olive-shaped burr covered with diamond crystals in its distal third that rotates at high speed and performs a differential cut when advancing through the vessel (figure 2A) while pulverizing calcified tissue and preserving the adjacent elastic tissue.13
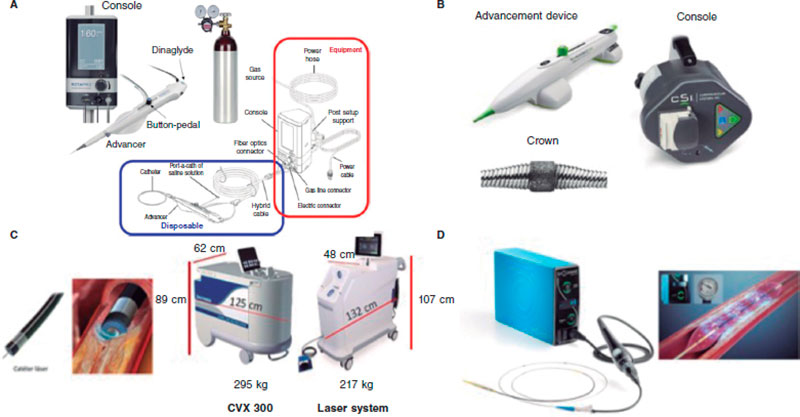
Figure 2. Plaque modification devices. A: Rotational atherectomy device. B: Orbital atherectomy device. C: Coronary laser device with the 2 existing console models. D: Intracoronary lithotripsy device. Modified with permission from Cubero-Gallego et al.13
It appeared over 30 years ago to facilitate the management of coronary artery lesions by reducing plaque burden. Early enthusiasm turned into an elevated use of RA in different settings without the proper scientific back-up. This triggered suboptimal results14 that reduced its use to highly selected cases only. Through all these years, RA has evolved with technological improvements, and also of the technique itself, as well as the selection of patients.
Currently, the ROTAPRO system (Boston Scientific, United States) is available. It has made the technique easier because it has replaed the pedal of the early version for a button placed on top of the olive-shaped burr advancer. There is another button on the side of the advancer to change to the Dynaglide mode (rotation at low revolutions is advised to introduce and remove the burr). Console is smaller and comes with a digital screen. Size of the burrs is between 1.25 mm and 2.5 mm, and they are compatible with 6-Fr-to-8-Fr catheters based on the size of the olive-shaped burr that advances on a 0.009 in specific guidewire (0.014 in the radiopaque side) of which 2 different versions exist (RotaWire Floppy and RotaWire Extra-Support) that should be used depending on the characteristics of the plaque and support needed.13
The main indication is to treat extremely calcified non-crossable or non-dilatable coronary artery lesions with balloon. Probably, the optimal scenario is a concentric calcified lesion with a smaller luminal area compared to the olive-shaped burr. Eccentric angulated lesions are less favorable since they are associated with a higher risk of complications.13,15 It can be used as a primary or a bailout strategy after “balloon failure”. The primary strategy has been associated with shorter procedures, less radiation and contrast, and probably lower cost regarding the material used.15
The target of RA has also changed from the old idea of removing as much plaque as possible (debulking) to the modern approach of modifying plaque to “facilitate” the PCI. Technical recommendations to perform RA have evolved too. Current recommendations are shown on table 3.16
Table 3. Recommendations for a safe use of rotational atherectomy
| Arterial access | It depends on the maximum size of the olive-shaped burr. Currently, the most widely used is radial access because it allows the use of burrs of up to 1.75 mm (when using a 6-Fr catheter) or 2.15 mm (when using a 7-Fr catheter) |
| Guide catheter | High-support catheters with a simple curve are advised |
| Guidewire | Direct guidewire placement is often feasible, although a conventional guidewire can be used, and exchange performed using a microcatheter or a coaxial balloon Based on the lesion characteristics, the RotaWire Floppy or Extrasupport can be used |
| Size of olive-shaped burr | The use of small burrs is advised to keep the burr/artery ratio ≤ 0.5. The size of the most widely used burr is 1.5 mm. In some cases, the gradual increase of the size of the burr is advised |
| Rotablation speed | Selection of rotablation speeds < 180 000 rpm—ideally between 135 000 rpm and 150 000 rpm—is advised. High speeds should be spared for cases where the burr cannot cross despite using the optimal technique available. Special attention should be paid to avoid drops > 5000 rpm during rotablation |
| Ablation time | Shorter ablation times (ideally ≤ 15 seconds) reduce the risk of complications (atrioventricular block, flow slowing down) |
| Ablation motion | Gradual, and continuous pecking motion |
| Cleansing serum | Heparinized saline solution should be used with vasodilators/spasmolytics (verapamil, nitrates) |
| Pacemaker | The use of olive-shaped burrs of smaller diameter, lower speeds, and the position of the burr with the Dynaglide mode have proven to reduce the number of transient atrioventricular blocks during rotablation substantially In selected cases, above all, in dominant right coronary or left circumflex arteries the preventive use of IV atropine or transient pacemaker implantation can be considered |
The most common complication is slow/no-flow although its rate has dropped down to 2.6%.17 It is due to debris embolization towards microcirculation and there is higher risk in long lesions where multiple and prolonged passes with large olive-shaped burrs are performed without proper pauses among them and in the presence of a poor distal vessel. The management of dominant right coronary or left circumflex coronary arteries can be associated with transient conduction disorders. Severe complications like burr entrapment, perforation, and coronary dissection are rare.13 Factors predisposing burr entrapment are lesion severity, steep angulations, and the use of very small burrs. Tortuosity and the lack of guide catheter coaxiality in the management of ostial lesions can trigger dissections and coronary perforations.
Although RA has demonstrated that it facilitates PCI with higher rates of success compared to balloon angioplasty, No clinical benefit has been yet confirmed.18-21
To analyze its results we should mention that RA has been used in patients of higher clinical risk with more complex coronary artery lesions.22 Another aspect we should take into consideration is the high percentage of cases where this technique was used as a bailout strategy (12% to 50%)20,21,23 meaning that without RA these cases would not have been performed or had had worse results. Although ongoing trials are studying the advantages of elective or bailout RA, proper patient and lesion assessment should lean towards increasing its elective or earlier use with a potential beneficial impact on clinical outcomes.24
In conclusion, when performed under the current recommendations RA is a safe and effective procedure. It should become part of our therapeutic arsenal in our cath labs with trained personnel for proper use.
Orbital atherectomy
The Diamonback-360 (OAS) device (Cardiovascular Systems, United States) is a diamond-coated bidirectional orbital crown that uses a combination of centrifugal force (by creating elliptical orbits) and friction to the surface to modify the calcified plaque and increase compliance (figure 2B). Also, with the pulsatile impact of the crown after speeding up, microfractures can occur that eventually modify deep calcium layers (figure 2B and figure 3). That is why a single 1.25 mm crown can treat vessels from 2.5 mm up to 4 mm.
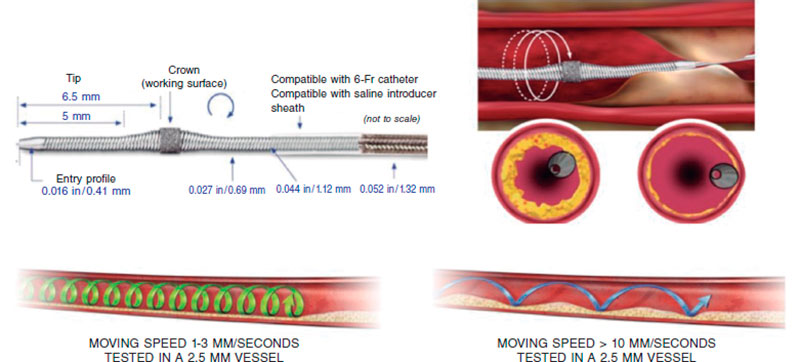
Figure 3. Characteristics of orbital atherectomy catheter and its effects. (Modified with permission from Cardiovascular Systems.25)
Compared to the remaining plaque modification techniques, this orbital atherectomy (OA) has arrived late to our country and our experience is still scarce.
Its main indication is to treat no-dilatable calcified coronary artery lesions.26
Preparation is very similar to RA, but here a specific guidewire is needed, the Viper-Wire. Crown advances with the Glide-Assist system (rotation at low revolutions) until coming close to the lesion. Another distinctive feature of this device is its antegrade and retrograde ablation functionalities. Unlike RA, the speed at which the device moves forward needs to be very slow (between 1 mm and 3 mm per second) to guarantee good procedural results and reduce complications.17,26 The mechanism of action of OA consists of the crown elliptical rotation that achieves a gradual increase of orbital diameter as rotation speed increases from 80 000 rpm up to 120 000 rpm. Cycles ≤ 30 seconds are advised (it comes with a sound warning signal to end the cycle) with pauses between 20 and 30 seconds among them that can duplicate in cases of poor hemodynamic tolerance.26 The continuous infusion of a lubricant solution is necessary to minimize thermal lesions during OA. Also, 18 mL/min are administered to cool the device down and get rid of debris, thus reducing the chances of ischemia and distal embolization.13,26,27
Complications are similar to those of RA. However, the possibility of retrograde application reduces the chances of crown entrapment and the risk of dissection or perforation in angulated or ostial lesions. The rate of perforation is between 0.7% and 2%.28,29 Theoretically speaking, the debris created by OA is smaller compared to the debris created by RA. This added to the fact that the crown does not stop coronary flow during atherectomy reduces the risk of slow/no-reflow and endothelial thermal lesion.27 However, transient conduction disorders are not rare when dominant right coronary or left circumflex arteries are treated.
Current evidence available is based on the ORBIT I30 and ORBIT II28 clinical trials where OA obtained good results regarding procedural success (94% and 89%, respectively) with higher rates of major adverse cardiovascular events (MACE), and target lesion failure of 23.5% and 7.8%, respectively, at 3 years.31 Afterwards, the COAST trial29 was conducted where the new MicroCrown system was used. A total of 100 patients were included with rates of procedural success and MACE of 85% and 22.2%, respectively, at 1-year follow-up. We are waiting to see the results from the ECLIPSE trial that will randomize a total of 2000 patients with severe calcifications to receive OA or balloon predilatation prior to by drug-eluting stent implantation.
In conclusion, OA is another calcium plaque modification technique with potential technical advantages like having only 1 size of crown compatible with 6-Fr to treat all lesions and with pull-back capabilities. Although there are insufficient data from comparative studies, choosing this technique will depend on the profile of the patient and the lesion to be treated, the intracoronary being an essential aspect.
Excimer laser
Excimer laser coronary angioplasty (ELCA) is based on a xenon chloride laser that generates short ultraviolet pulses of 308 mm that only penetrate 50 µm in depth, which makes it safer compared to old continuous-wave-near-infrared lasers. It modifies the plaque through a triple mechanism: photochemical (by breaking molecular binds), photothermal (through tissue vaporization), and photokinetic (through the expansion and collapse of the bubble of the catheter tip as it moves forward). Fragments released are < 10 μm, which minimizes microvascular damage after being absorbed by the reticuloendothelial system.
The system currently used is the CVX-300 Laser System (Philips) although there is already a new generation one, the LAS-100 Laser System (Philips) that will be replacing it shortly (figure 2C). There are different sizes of catheters available (0.9 mm, 1.4 mm, 1.7 mm, and 2.0 mm) (table 4). The selection of the catheter depends on the type of lesion and size of the vessel (catheter to vessel diameter ratio, 0.5-0.6) being the 0.9 mm catheter the most widely used for its lower profile and because it can reach higher fluency (80 mJ/mm2), pulse repetition rate (80 Hz), and longer application durations (10 seconds with 5-second rests), which increases the chances of success in fibrous calcific plaques.32,33
Table 4. Characteristics of Excimer laser coronary angioplasty catheters
| 0,9 mm-X 80 | 1,4 mm | 1,7 mm | 2 mm | |
|---|---|---|---|---|
| Compatible guide catheter | 6-Fr | 6-Fr/7-Fr | 7-Fr | 8-Fr |
| Minimum vessel diameter (mm) | 2 | 2.2 | 2.5 | 3 |
| Energy (mJ/mm2) | 30-80 | 30-60 | 30-60 | 30-60 |
| Frequency (Hz) | 25-80 | 25-40 | 25-40 | 25-40 |
| Application/pause time (seconds) | 10/5 | 5/10 | 5/10 | 5/10 |
Before being used, it is necessary to calibrate the console and then the catheter. In both cases, health professionals and patients should use protective glasses to prevent eye damage. Afterwards, a 0.014 in intracoronary guidewire is inserted until it reaches the lesion. There is a monorail system to facilitate moving forward. Energy is released through the catheter distal border as it slowly advances (0.5 mm/second) to modify the plaque. Catheter withdrawing can also be applied. It is important to optimize support to ensure that the catheter advances. There is no limit in the number of pulses that can be administered since the more it is used, the stronger the effect. However, there is also a higher risk of complications involved. Some authors suggest a maximum of 12 applications.33 The state of the vessel should be assessed after each application. Regarding the selection of parameters, traditionally it started at 45 mJ/mm2, and 25 Hz. However, more and more operators prefer higher energies and initial frequencies especially to treat resistant or calcified lesions.33
Before and during the applications, the blood vessel should be washed, and contrast administered through the infusion of a physiological saline solution (1 mL/s to 3 mL/s). In resistant lesions with severe calcification or underexpanded stents, more energy may be needed. This can be reached by not washing the blood with the physiological saline solution or even administering contrast during applications (the so-called “explosion technique”). This technique reaches maximum power although it increases the chances of complications. Some authors33 recommend it as the first option to treat non-thrombotic lesions, although it seems reasonable to spare it for ELCA-resistant lesions with saline infusion.
Traditionally, the indications for ELCA have been categorized into 2 different groups: “thrombotic” (not discussed in this document) and “calcified” lesions (non-thrombotic like in-stent restenosis, chronic total coronary occlusions, calcified lesions, etc.). The latter can be categorized into non-crossable or non-dilatable lesions:
Non-crossable lesions
The laser main advantage is that it is compatible with all intracoronary guidewires. Therefore, non-crossable lesions with balloon/microcatheter are its main indication.17 In a multicenter registry of non-crossable lesions, the rate of procedural success was 87.3% with 0.8% of dissections showing an impaired flow and no perforations.34 Severe calcification has been associated with a higher probability of technique failure34 since ablation is primarily performed in the tissues between calcium.35 However, the use of ELCA with contrast can increase its chances of success in these lesions.33
Non-dilatable lesions
Although the success of ELCA in non-dilatable lesions is high,36 it has never been the first-line therapy. Among these lesions, an interesting scenario is in-stent lesions (restenosis or underexpansion). In acute underexpansion, ELCA could be the treatment of choice. It allows the modification of resistant tissue located behind the stent without changing its architecture. Its use with contrast can be safer thanks to the stent “protective” effect. Isolated cases and small case series with success rates > 95% and few complications have been published.37
It is a safe technique when the recommendations given are observed. Coronary artery dissection is the most common complication (5), although it is rarely flow-limiting (1%). The rate of coronary artery perforation is < 1%,38 and distal embolizations and ventricular arrhythmias are exceptional.39
In conclusion, ELCA is especially useful to treat non-crossable lesions thanks to its compatibility with all kinds of angioplasty guidewires. It has also proven effective to treat non-dilatable lesions including in-stent lesions. However, there is still scarce information on its efficacy in calcified coronary artery lesions.
BALLOON-BASED TECHNIQUES
Cutting and scoring balloons
Cutting balloons (CB) are plaque modification devices that appeared as an alternative to old coronary angioplasty balloons.40 Their objective is to achieve controlled ruptures of the plqeu (through incisions in fibrocalcific tissue) (figure 4), thus facilitating balloon expansion, minimizing damage to the intima layer, and reducing stenosis.18,41
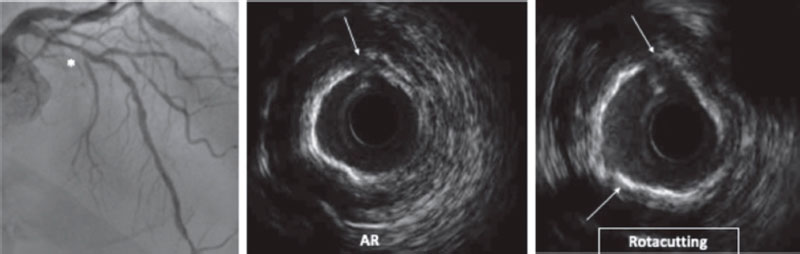
Figure 4. Rotacutting technique. Rotational atherectomy (RA) effect and cutting balloon (Rotacutting) with greater plaque modification and minimum lumen area.
There are 2 different types: CB and scoring balloon (BS). Their use has been described in different settings like in-stent restenosis, aorto-ostial lesions, bifurcations, and small vessels associated with the use of drug-eluting stents.42
The main limitations of CBs are their worst navigability and crossing profile compared to conventional balloons. However, over the past few years, both aspects have improved. SBs are associated with better navigability compared to old CBs.
The most dreaded complication is the rupture of coronary artery, although it has significantly increase following its use.
The main difference among the different devices lays in their different external atherotomy elements as described herein (figure 5).
Figure 5. Characteristics of modified balloons. A: Cutting balloon (Boston Scientific, United States). B: WOLVERINE (Boston Scientific, United States). C: AngioSculpt (Spectranetics, United States). D: Scoreflex (OrbusNeich, Hong Kong). E: Grip (Acrostak, Switzerland). F: NSE-Alpha (B.Braun, Germany). G: Naviscore (iVascular, Spain).
Cutting Balloon Flextome
Cutting Balloon Flextome (Boston Scientific, United States) consists of a noncompliant (NC) balloon with 3 microrazors longitudinally mounted on the surface. Its superiority over conventional balloons in A/B lesions has not been confirmed yet, which is why, so far, its use is limited to complex17 and calcified lesions only.43
WOLVERINE
Wolverine (Boston Scientific, United States) is an evolution of the former one with a better crossing profile, greater flexibility, and a more visible tip.
AngioSculpt
AngioSculpt (Spectranetics, United States) is a semicompliant balloon with low crossing profile surrounded by 3 nitinol filaments arranged in a helical cage to secure balloon anchorage. There is a lower risk of dissection or perforation associated with this device.17 It provides more flexibility and better navigability compared to old CBs,44 as well as good results compared to dilatation with semicompliant balloons.45
Scoreflex
Scoreflex (OrbusNeich, Hong Kong) is a NC consisting of a NC balloon with a nitinol dual-wire system to facilitate the controlled modification of the plaque at low pressures. It has a low profile and a combination of hydrophilic and hydrophobic coating that minimizes friction during lesion crossing.
Grip
Grip (Acrostak, Switzerland) is a high-pressure balloon with 4 rows of 3 or 4 knobs in each row. It allows dilatations of up to 22 atm. It comes with a cone-shaped tip in 2 different versions: Grip with a short 2 mm tip, and Grip TT with a long 4 mm tip for greater navigability in tortuous anatomies. It comes with a hydrolubricated coating on its tip and the catheter (not on the balloon), which facilitates both its anchorage to the lesion and navigability across lesions.
NSE Alpha
NSE Alpha (B. Braun, Germany) is a SB with 3 nylon scoring elements and 1 triangular cutting section connected in both borders of the balloon and arranged in a 120º disposition. We should mention its flexibility and navigability with good results in de novo lesions and in-stent restenosis.18
NaviscoreTM (iVascular, Spain)
NaviscoreTM (iVascular, Spain) is a SB with a design that combines the benefits of SB plus CB. It consists of a high-pressure balloon with 125 μm nitinol filaments. These have an axial orientation for greater crossing abilities and flexibility, and plaque modification in a 90º angle, which is associated with lower chances of perforation. The catheter hydrophilic coating improves its navigability.
In conclusion CBs and SBs are useful plaque modification devices to treat non-dilatable lesions when calcification is not very severe. Their main advantage is how easy they are to use since it is a balloon-based technique compatible with conventional angioplasty guidewires.
Very high-pressure balloons
The NC very high-pressure balloon (VHPB) OPN (SIS medical, Switzerland) is a double-layer balloon for homogeneous expansion at extremely high pressures without increasing its diameter (from 2 mm to 4 mm) with a rated burst pressure of 35 atm, although the manufacturer’s testing rated burst pressure limit is 45 atm (table 5).46
Table 5. Compliance of the NC very high-pressure OPN balloon
| Pressure (atm) | NC OPN 2.0 (mm) | NC OPN 2.5 (mm) | NC OPN 3.0 (mm) | NC OPN 3.5 (mm) | NC OPN 4.0 (mm) |
|---|---|---|---|---|---|
| 10 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| 20 | 2.1 | 2.6 | 3.14 | 3.67 | 4.19 |
| 30 | 2.18 | 2.7 | 3.29 | 3.85 | 4.37 |
| 35 | 2.2 | 2.77 | 3.36 | 3.91 | 4.41 |
NC, noncompliant. | |||||
The VHPB has been used for over 10 years now and it has proven safe and effective in up to 40 atm in extremely calcified coronary artery lesions where other devices have failed or in stent underexpansion. Success rates are as high as 75% to 100% without evidence of dissection, perforation or balloon bursts in small case series.47 Compared to conventional NC balloon, it can achieve minimum luminal diameters and major acute gains with less residual stenosis in non-dilatable lesions.48
The largest registry ever conducted to this date included 326 patients with non-dilatable lesions treated with VHPB after failed NC balloon. Patients were categorized into 2 groups: those who responded to pressures between 30 atm and 40 atm, and those who responded with pressures > 40 atm. Procedural success was reached in up to 96.6% of the patients. A total of 53% of the patients responded to pressures between 30 atm and 40 atm while the remaining 47% did so to pressures > 40 atm. A total of 180 patients were treated with intracoronary imaging modalities and 106 of these showed calcifications > 270º. In this subgroup of patients, the pressured needed for optimal expansion was > 40 atm in 78.3% of the cases. Three patients (0.9%) showed coronary artery ruptures that resolved with prolonged inflation or covered stent implantation. In the 3 cases, the ruptures occurred during predilatation and were associated with balloon bursts with pressures between 30 atm and 40 atm. This is suggestive that perforations don’t seem to be associated with inflation pressure but with the characteristics of the plaque or the vessel size estimate that was angiographic in the 3 cases reported.49
The ISAR-CAL trial50 was published back in 2021. It randomized 70 patients with extremely calcified coronary artery lesions and failed predilatation with NC balloon to receive a SB or a VHPB. The study primary endpoint was to compare stent expansion on the OCT. No differences were reported in the percentage of stent expansion. However, differences were seen in angiographic secondary endpoints like improved minimum luminal diameters and residual stenoses favorable to VHPB.50
Finally, chronic total coronary occlusions are the pinnacle of calcified complex lesions. In the PLACCTON trial the use of the VHPB both alone and with other plaque modification techniques was safe and effective in selected cases with chronic total coronary occlusions.51
In conclusion, the VHPB is a safe and effective alternative to treat non-dilatable calcified coronary artery lesions. Randomized clinical trials better defining this device strategy of use and the remaining plaque modification techniques are lacking.
Intracoronary lithotripsy
Intracoronary lithotripsy (ICL) system consists of a specific balloon catheter (Shockwave Medical, United States) connected to a rechargeable portable generator (figure 2D). The generator produces energy pulses that are transmitted to emitters placed inside the balloon. Pulses are emitted at a frequency of 1 per second up to a maximum of 10 pulses per application. Each balloon catheter can administer a maximum of 80 pulses. The catheter consists of a rapid-exchange semicompliant balloon with a 0.042 in crossing profile compatible with any 0.014 guidewires and 6-Fr guide catheters.
Its main indication is to treat calcified non-dilatable coronary artery lesions.
A 1:1: ratio between the vessel and balloon diameters is advised. Once it has been placed inside the lesion, the balloon inflates at 4 atm to secure proper contact between the balloon surface and the vascular wall to allow energy transfer. The balloon includes 2 emitters that receive an electric discharge from the generator that vaporizes the fluid inside generating sound waves that have a local effect. Each pulse releases the equivalent of 50 atm.
These waves run across soft tissues causing selective calcium microfractures at intima and media layer level. After pulse emission and the corresponding modification of calcium, the balloon inflates up to 6 atm to maximize luminal gain. Balloon catheter is only available at a length of 12 mm and comes in diameters of 2.5 mm, 30 mm, 3.5 mm, and 4.0 mm.52
The greatest evidence available comes from the Disrupt-CAD III trial, a prospective registry that assessed the efficacy and safety profile of ICL in 431 patients with calcified lesions. The 30-day rate of MACE (death, infarction or target lesion revascularization) was 7.8% while the rate of effectiveness (procedural success with in-stent stenosis < 50%) was 92.4%. No patients with acute myocardial infarction or complex lesions were included in this study.53 Recently, 12-month follow-up results have been published confirming rates of MACE and stent thrombosis of 13.8% and 1.1%, respectively.54
Controlled break down of coronary calcium is the basis of treatment of ICL balloons. In a OCT substudy of the Disrupt-CAD II trial after ICL calcium fractures were seen in 79% of the lesions55 compared to 67% of the lesions of the Disrupt-CAD III trial.53
Although the use of ICL balloons has become very popular worldwide, information on its safety and efficacy profile regarding its use in complex settings is still scarce (acute coronary syndrome, chronic total coronary occlusions, bifurcations or aorto-ostial lesions). As a matter of fact, its use is often limited to isolated cases or short series.52 The main limitations of this system are its reduced crossing profile in extremely calcified or tortuous stenoses and difficult use in diffuse or multivessel lesions (due to the limited number of pulses per catheter and the different caliber of target vessels).
A recent trial assessed the use of underexpanded stents due to severe coronary artery calcification and confirmed angiographic success rates of up to 73%, which is lower compared to the 75% seen in native lesions56 probably because it is more difficult to expand a calcified lesion when the stent has already been deployed. Therefore, regardless of the technique used, stenting is ill-advised until the lesion has been properly prepared. Also, the application of lithotripsy in this context, especially on freshly implanted stents, can cause structural damage to the polymer.57 Another multicenter registry proved the device was successful 92.3% of the times in this type of lesions.58 Mid- and short-term data on the safety profile of this technique are still lacking.
The combined use of ICL balloon and other plaque modification devices like RA,59 OA60 or ELCA61 has been described, and it seems like a very attractive strategy in cases where the ICL balloon cannot reach the target lesion.
In conclusion, ICL has grown exponentially in the management of non-dilatable calcified coronary artery lesions thanks to its safety and efficacy profile, and short learning curve. However, information on its use in complex scenarios and comparative results with other plaque modification techniques are still lacking.
COMBINED TECHNIQUES
There is not much evidence on the combination of devices or plaque modification techniques in extremely calcified coronary artery lesions.
The use of RA followed by CB (RotaCutting) (figure 4) or lithotripsy (RotaTripsy) (figure 6) has been described as an additional, safe, and effective technique.62-64 In both cases the concept is similar. Primarily, RA damages superficial calcium, but not the deepest calcium layers, and there are times when it is not enough for proper plaque preparation. On the other hand, CB or lithotripsy can complement the plaque modification provided by RA. However, when calcified lesions progress into very severe aortic stenosis, the target lesion can be difficult to reach with these balloons. In a combined use, RA modifies superficial calcium by creating a tunnel that the CB or lithotripsy balloon can use to move forward and, when in position, complete plaque modification. One of the differences between both techniques is that CB can contribute to breaking down the calcium layer in the absence of very severe calcification. The RotaTripsy technique59,63 can be more effective to treat extremely calcified coronary artery lesions with thick calcium layers. However, its cost is also higher. Based on a similar concept, the combination of OA plus lithotripsy has been recently described with good results.60
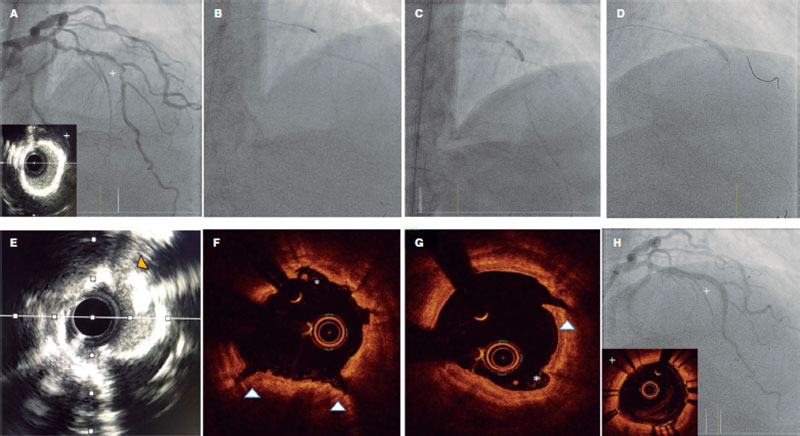
Figure 6. Rotatripsy technique. A: Extremely calcified stenosis in left anterior descending coronary artery. Intravascular ultrasound (IVUS) with 360º calcification. B: Rotational atherectomy (RA) (1.5 mm olive-shaped burr). C: 3 mm noncompliant balloon underexpansion after RA. D: Intracoronary lithotripsy with 3 mm balloon and proper expansion at 6 atm after 50 pulses. E-G: IVUS and optical coherence tomography showing the combined effect of RA plus lithotripsy with multiple zones of intimal “sanding/dissection” caused by the RA (asterisks), and deep and intimal fractures caused by lithotripsy. H: Final outcomes after stenting.
RA has also teamed up with ELCA (the RASER technique).65 Laser can be the only option in truly non-crossable lesions to facilitate the advancement of a microcatheter, perform the RotaWire exchange, and complete the PCI. This can also be used similarly by combining laser plus OA.
The combination of ELCA plus lithotripsy (the ELCATripsy technique) has been described for cases where RA or OA are ill-advised like nearby lesions or at freshly implanted stent level. In these cases, laser can create a tunnel through which the lithotripsy balloon can advance without the risk or damaging the freshly implanted stent.61
ALGORITHM FOR THE OPTIMAL MANAGEMENT OF CALCIFIED CORONARY ARTERY LESIONS
To select the most suitable plaque modification technique we need to become familiar with the characteristics of the different techniques available, their indications, and risks (table 6). Also, the patient’s clinical profile should be taken into consideration, as well as the characteristics of the lesion, the resources available, and the operator’s skills. In some complex cases, it can be reasonable to perform an ad hoc PCI for proper planning and even an angioplasty between 2 expert operators.
Table 6. Comparison of the different plaque modification techniques available
| Techniques non derived from balloon technology | Techniques derived from balloon technology | ||||||
|---|---|---|---|---|---|---|---|
| RA | OA | ELCA | CB | SB | VHPB | ICL | |
| Technical characteristics | |||||||
| Description of the technology involved | Diamond-coated olive-shaped burr rotating at high speed | Diamond-coated crown with elliptical rotation | Ultraviolet energy with photochemical, photothermal, and photokinetic effects | NC balloon with longitudinal microrazors | SC balloon with scoring elements on its surface | Double-layer NC balloon to allow very high-pressures | SC balloon emitting pulsatile mechanical energy |
| Mechanism of action | Differential cut/Antegrade abrasion. Additional effect from crown vibration (+) | Differential sanding/ Antegrade and retrograde abrasion. Additional effect from crown vibration (+++) | Photoablation/vaporization | Superficial cut of the plaque | Superficial cut of the plaque | Inflation at 35 atm to 40 atm | Lithotripsy/Calcium fragmentation |
| Size of devices | 1.25 mm to 2.5 mm burr | 1.25 mm crown | 0.9 mm to 2 mm catheters | 2 mm to 4 mm | 1.47 mm to 4 mm | 1.5 mm to 4 mm | 2.5 mm to 4 mm |
| Compatible GC* | 6-Fr; 1.25 mm and 1.5 mm 7-Fr; 1.75 mm 8-Fr; 2.0 mm and 2.15 mm 9-Fr; 2.25 mm and 2.38 mm 10-Fr; 2.50 mm | 6-Fr | 6-Fr: 0.9 mm and 1.4 mm 7-Fr: 1.7 mm 8-Fr: 2.0 mm | 6-Fr | 6-Fr (some with 5-Fr) | 6-Fr | 6-Fr |
| Type of compatible guidewire | 0.009 in RotaWire (0.014 in the radiopaque part) | 0.012 in ViperWire (0.014 in the radiopaque part) | Any 0.014 in guidewire | Any 0.014 in guidewire | Any 0.014 in guidewire | Any 0.014 in guidewire | Any 0.014 in guidewire |
| Type of Console/System | Small without pedal (RotaPro) | Small without pedal | Large with pedal | – | – | – | Small without pedal |
| Indications and effects | |||||||
| Main indication | Plaque modification (non-dilatable calcified coronary lesions or only crossable through microcatheter) | Plaque modification (non-dilatable calcified coronary lesions or only crossable through microcatheter) | Plaque modification (non-crossable lesion, in-stent non-dilatable coronary lesions) | ISR | ISR | Optimization of stent expansion | Calcified plaque modification |
| Effect on intimal or deep calcium layers | Intimal | Intimal and deep | Intimal and deep | Intimal | Intimal | Intimal | Intimal and deep |
| ISR | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Stent underexpansion | Chronic only | Chronic only | Acute or chronic | – | – | Acute or chronic | Recommended in chronic only |
| Advantages | Useful in non-crossable lesion with balloon Greater availability compared to OA/ELCA | Possibility of retrograde application (useful in angulated/ostial lesions) 1 crown only for all cases (compatible with 6-Fr) | No need for specific guidewire. 0.9 mm catheter (the most common one) compatible with 6-Fr Of choice in non-crossable lesion with balloon and microcatheter It allows the use of guidewires in the side branches | Short learning curve. Compatible with 0.014 in and 6-Fr guidewires It allows the use of guidewires in the side branches. Lower cost | Short learning curve. Compatible with 0.014 in and 6-Fr guidewires It allows the use of guidewires in the side branches Lower cost | Short learning curve. Compatible with 0.014 in and 6-Fr guidewires It allows the use of guidewires in the side branches Lower cost | Short learning curve. Compatible with 0.014 in and 6-Fr guidewires It allows the use of guidewires in the side branches |
| Disadvantages | Long learning curve Need for specific guidewire Need for large French sizes for large burrs | Long learning curve Need for specific guidewire Worse crossing ability in non-crossable lesions with balloon It requires a specific lubricant contraindicated in patients allergic to egg and soybean | Intermediate learning curve Large console and need for warming up/calibration | Limited crossing ability Useless in extremely severe calcifications | Useless in extremely severe calcifications | Limited crossing ability | Limited crossing ability Limit per catheter pulses |
| Complications | |||||||
| Major perforation/dissection | Moderate | Moderate | Moderate | Low/moderate | Low/moderate | Low/moderate | Low |
| Slow/No-Flow | Moderate | Moderate | Low | Low | Low | Low | Low |
| Atrioventricular block | Moderate in dominant RCA/LCx | Moderate in dominant RCA/LCx | Low | Low | Low | Low | Low |
| Entrapment | Moderate (greater with 1.25 mm burrs and severe angulated lesions) | Low | Low | Low | Low | Low (entrapment over the guidewire is not rare; consider second parallel guidewire) | Low |
| Technical recommendations | Speeds of 135 000 rpm to 180 000 rpm Device/vessel ratio ≤ 0.6. Pecking motion Short cycles with pauses among them. Avoid angulated lesions | Speeds of 80 000 rpm to 120 000 rpm Slow continuous forward and backward motion (useful in angulated/ostial lesions) Short cycles with longer pauses among them if hemodynamic impairment Avoid antegrade access in angulated lesions | Device/vessel ratio ≤ 0.6 Slow continuous forward motion (also applicable in backward motion) Application during the injection of a saline solution Application without washing or with saline solution or contrast injection in selected cases Avoid angulated lesions | Balloon-artery ratio 1:1 Slow and gradual inflation and deflation Balloon rotation followed by repeat inflations can increase the number of incisions | Balloon-artery ratio 1:1 Slow and gradual inflation and deflation | Balloon-artery ratio 1:1 Slow and gradual inflation and deflation | Balloon-artery ratio 1:1 Optimal balloon air purge Inflation sequence at 4 atm, application of 10 pulses, and inflation at 6 atm Gradual deflation after console beeping At least 20 pulses per lesion |
CB, cutting balloon; ELCA, Excimer laser coronary angioplasty; GC, guide catheter; ICL, intracoronary lithotripsy; ISR, in-stent restenosis; LCx, left circumflex artery; MC, microcatheter; NC, noncompliant; OA, orbital atherectomy; RA, rotational atherectomy; RCA, right coronary artery; SB, scoring balloon; SC, semicompliant; VHPB, very high-pressure balloon. | |||||||
Current evidence available from comparative or clinical trials allowing us to select among the different plaque modification techniques available is very limited66,67 (table 7). Therefore, although several algorithms have been proposed on the type of calcium and the plaque modification technique that should be used,67 there are no clear indications in the routine clinical practice guidelines. Currently ongoing studies may bring us more in-depth information in the future.
Table 7. Main clinical trials on plaque modification techniques
| Trial (year) | Design and sample size | Type of lesion | Main results |
|---|---|---|---|
| Rotational atherectomy | |||
| ROTAXUS20,24 (2013) | RCA of 240 p. (120 RA: 120 ST) | Moderate to severe calcification | – Successful strategy: RA, 92.5% vs ST, 83.3%; P = .03 – Acute luminal gain: RA, 1.56 mm vs ST, 1.44 mm; P < .01 – No significant differences regarding dissections, perforations or slow/no-reflow – Stent luminal loss at 9 months: RA, 0.44 mm vs ST, 0.31 mm; P = .04 – MACE at 9 months: RA, 24.2% vs ST, 28.3%; P = .46 – MACE at 2 years: RA, 29.4% vs ST, 34.3%; P = .47 |
| PREPARE CALC21 (2018) | RCA of 200 p. AR vs MB (cutting or scoring) | Severe calcification | – Successful strategy: RA, 98% vs MB, 81%; P = .0001 – No significant differences regarding dissections, perforations or slow/no-reflow – Luminal loss at 9 months: RA, 0.22 mm vs MB, 0.16 mm; P = .21 – TLR at 9 months: RA, 2% vs MB, 7%; P = .17 – No significant differences at 9 months regarding mortality or stent thrombosis |
| Orbital atherectomy | |||
| ORBIT I30 (2013) | NRPT of 50 p | Calcification (mild to severe) | – Procedural success (residual stenosis <20% after stenting): 94% – Rate of MACE at 6 months: 8% – Dissection: 12% – Perforation: 2% |
| ORBIT II28,31 (2014) | NRPT of 443 p | Severe calcification | – Procedural success (stenosis < 50% after stenting without in-hospital MACE): 98.6% – Severe dissection: 2.3% – Perforation: 0.9% – Slow/no-reflow: 0.2%M – MACE at 30 days and 3 years: 10.4% and 23.5%, respectively |
| COAST29 (2020) | NRPT of 100 p | Severe calcification | – Procedural success (stenosis < 50% after stenting without in-hospital MACE): 85% – Dissection: 2% – Perforation: 2% – Slow/no-reflow: 2% – MACE at 30 days and 1 year: 15% and 22.2%, respectively |
| ELCA | |||
| Fernandez et al.36 (2013) | Observational trial of 58 p | – Balloon failure (non-crossable or non-dilatable lesions) treated with ELCA ± RA – Calcification > moderate: 82.1% | – Procedural success (stenosis < 20% after stenting without flow-limiting dissection or type II or III perforations): 91% – ELCA success isolated in 76.1%; ELCA after failed RA, 6.8% and ELCA + RA, 8.6% – Only 1 successful case of RA when ELCA failed – 4 procedural complications reported (1 transient slow flow, 1 side branch occlusion, and 2 perforations) |
| ELLEMENT37 (2014) | Observational trial of 28 p | – Stent underexpansion treated with high-energy ELCA with contrast after NC balloon failure – Calcification: 89.3% | – Laser success (increase ≥ 1 mm2 in SMA with IVUS or ≥ 20% MLD on the quantitative coronary angioggraphy after predilatation with the NC balloon that failed before ELCA): 96.4% – Perioperative infarction: 7.1% – Transient slow flow: 3.6% |
| LEONARDO68 (2015) | Observational trial of 100 p | – Balloon failure in complex lesions – Calcification: 57%. | – Procedural success (stenosis <50% after stenting): 91.7% – No perforations, dissections, significant side branch occlusions, spasms or lack of flow |
| LAVA69 (2018) | Observational trial of 130 lesions | – Non-crossable lesions with balloon: 43.8% – Non-dilatable lesions with balloon: 40.8% – Moderate or severe calcification: 62% – ISR: 37% | – Procedural success: 88.8% (93.8% in non-dilatable lesions and 83.7% in non-crossable lesions) – Perforation: 1.78% – Perioperative infarction: 0.86% |
| Ojeda et al.34 (2020) | Observational trial of 126 lesions | – Non-crossable lesions with balloon – Calcification ≥ moderate: 62.7% – Chronic total coronary occlusion: 46% | – Technical success (residual stenosis < 30% and TIMI grade-3 flow): 90.5% – Procedural success (technical success without in-hospital adverse events): 87.3% – Severe calcification associated with failed ELCA |
| Modified balloons (cutting or scoring balloons), and VHPB | |||
| ISAR-CALC50 | RCA of 74 p (VHPB vs SB) | – Extremely calcified non-dilatable lesions with balloon | – Stent expansion on the CTO similar compared to VHPB and SB (0.72 ± 0.12 vs 0.68 ± 0.13; P = .22) – VHPB: higher increase of MLD (2.83 mm ± 0.34 mm vs 2.65 mm ± 0.36 mm; P = .03) and less stenosis (11.6% ± 4.8% vs 14.4% ± 5.6%; P = .02) – No differences associated with procedural success |
| Intracoronary lithotripsy | |||
| DISRUPT CAD III53,54 | NRPT of 431 p | Severe calcification | – Procedural success (residual stenosis < 50% without in-hospital MACE): 92.4% – Perioperative infarction 6.8% – Severe dissection: 0.3% – Perforation: 0.3% – Slow or no-reflow 0% – TLR at 30 days: 1.3% – Stent thrombosis: 0.8% – MACE at 1 year 13.8% |
ELCA, Excimer laser coronary angioplasty; ISR, in-stent restenosis; IVUS, intravascular ultrasound; MACE, major adverse cardiovascular events; MB, modified balloon; MLD, minimum luminal diameter; NC, noncompliant; NRPT, non-randomized prospective trial; OCT, optimal coherence tomography, p, patients; QCA, quantitative coronary angiography; RA, rotational atherectomy; RCA, randomized clinical trial; SB, scoring balloon; SMA, stent minimal area; ST, standard therapy; TIMI, Thrombolysis in Myocardial Infarction; TLR, target lesion revascularization; VHPB, very high-pressure balloon. | |||
In cases of mild angiographic calcification and proper balloon expansion, further plaque preparation prior to stenting may not be required. However, when angiographic calcification is moderate or severe, the use of intracoronary imaging modalities is advised for their great utility to plan the procedure and optimize results (figure 7).
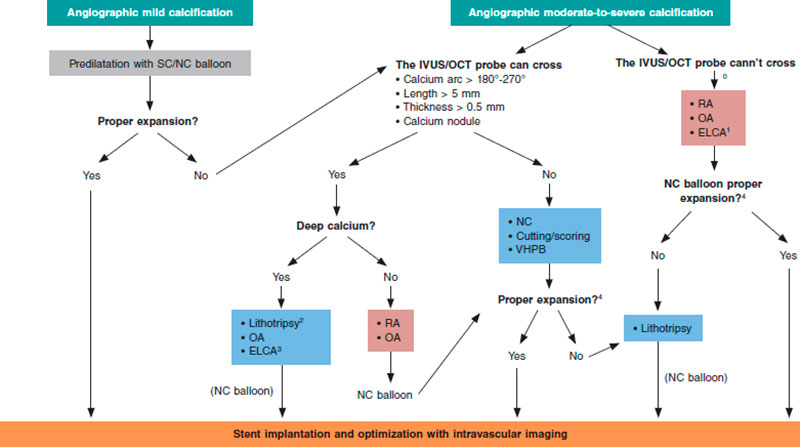
Figure 7. Central figure. Calcified plaque modification algorithm. ELCA, Excimer laser coronary angioplasty; NC, noncompliant; OA, orbital atherectomy; RA, rotational atherectomy; SC, semicompliant; VHPB, very high-pressure balloon.
0 Predilatation with low-profile balloons can be attempted. At times, this allows early assessment with intravascular imaging tools.
1 Of choice if microcatheter is unable to cross.
2 If lithotripsy balloon is unable to cross, predilatation can be attempted with balloon or combination of other techniques (Rotatripsy, Elcatripsy, Orbital-tripsy).
3 Currently, lithotripsy is preferred in the presence of acute stent underexpansion.
4 In addition to NC balloon final angiographic expansion, intracoronary imaging are useful to confirm the effect of the techniques used on plaque modification.
Overall, it is useful to apply the “rule of 5”: lesions where calcium occupies < 50% of arc circumference (180º), does not extend longitudinally > 5 mm, and thickness is not > 0.5 mm can be properly treated with high-pressure or modified balloons (CB or SB).
If these criteria are met or calcium nodules are spotted further advanced plaque modification techniques should be used. In addition to circumferential and longitudinal spread, and thickness, calcium depth is important as well since some techniques like RA act basically on the superficial—and not on the deep—portion of calcium plaque.
Lesions with extremely severe calcifications so stenotic that cannot be crossed with the IVUS or OCT probe probably need RA/OA or laser (that can be of choice if the lesion is non-crossable not even with a microcatheter to allow specific RA/OA guidewire exchange). Another alternative is to try predilatation with low-profile balloons that often allow early assessments with intravascular coronary imaging to guide the decision-making process as already described.
Balloon expansion after using these techniques will guide us on proper plaque preparation. Also, intracoronary images are very useful to confirm proper calcium modification to allow stent expansion. The effects of different techniques like the presence of fractures (with balloon or lithotripsy), superficial calcium sanding (with RA) or both effects (with OA)70 can be visible when intracoronary imaging modalities are used (figure 6). After the use of ELCA, superficial and deep fractures have been described. However, effects may not be visible on the OCT and, same as it happens with ICL, that does not mean that the plaque has not been modified.
Based on the type of lesion and effects caused by these techniques, the combination of 1 or more of these techniques can be necessary to secure optimal stenting and favorable clinical outcomes.
CONCLUSIONS
Coronary artery calcification is probably the greatest determinant of poor PCI outcomes and incomplete percutaneous revascularizations, and is associated with higher rates of adverse events. Intracoronary imaging modalities play a key role in the understanding of calcified coronary artery lesions, help us select the plaque modification technique we’ll eventually use, and optimize the PCI results. Knowing the different plaque modification techniques available is essential for the optimal management of calcified coronary artery lesions. Until comparative trials among techniques are conducted, it seems reasonable to combine them depending on the type of lesion. In addition, there are situations in which techniques should be combined to secure optimal stenting and the most favorable clinical outcomes.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
Manuscript drafting: A. Jurado-Román, A. Gómez-Menchero, N. Gonzalo, J. Martín-Moreiras, R. Ocaranza, Soledad Ojeda, J. Palazuelos, O. Rodríguez-Leor, P. Salinas, B. Vaquerizo, X. Freixa, and A.B. Cid-Álvarez. Design, coordination, review process of the final version, and manuscript submission: A. Jurado-Román.
CONFLICTS OF INTEREST
S. Ojeda is an associate editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. A. Jurado-Román, X. Freixa, and A. B. Cid-Álvarez are members of the ACI-SEC board of directors.
REFERENCES
1. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc Imaging. 2018;11:127-142.
2. Wang X, Matsumura M, Mintz GS, et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovasc Imaging. 2017;10:869-879.
3. Copeland-Halperin RS, Baber U, Aquino M, et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES: Findings from a large multiethnic registry. Catheter Cardiovasc Interv. 2018;91:859-866.
4. Sharma SK, Bolduan RW, Patel MR, et al. Impact of calcification on percutaneous coronary intervention: MACE-Trial 1-year results. Catheter Cardiovasc Interv. 2019;94:187-194.
5. Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189.
6. Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961-972.
7. Kawashima H, Serruys PW, Hara H, et al. 10-Year All-Cause Mortality Following Percutaneous or Surgical Revascularization in Patients With Heavy Calcification. JACC Cardiovasc Interv. 2022;15:193-204.
8. Hendry C, Fraser D, Eichhofer J, et al. Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. EuroIntervention. 2012;8:79-86.
9. Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959-1965.
10. Zhang M, Matsumura M, Usui E, et al. Intravascular Ultrasound-Derived Calcium Score to Predict Stent Expansion in Severely Calcified Lesions. Circ Cardiovasc Interv. 2021; e010296.
11. Stefanini GG, Alfonso F, Barbato E, et al. Management of Myocardial Revascularization Failure: An Expert Consensus Document of the EAPCI. EuroIntervention. 2020;16:e875-e890.
12. Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14:656-677.
13. Cubero-Gallego H, Tizón-Marcos H, Vaquerizo B. Opciones actuales para el tratamiento de las lesiones calcificadas. REC Interv Cardiol. 2020;2:129-139.
14. Hellig F, Pandie S, Barbato E, Colombo A, Heyndrickx JR. Rotational atherectomy. En: PCR - EAPCI Textbook 2015, Part III. Europa Digital & Publishing; 2015.
15. Sharma SK, Tomey MI, Teirstein PS, et al. North American Expert Review of Rotational Atherectomy. Circ Cardiovasc Interv. 2019. https://doi.org/10.1161/CIRCINTERVENTIONS.118.007448.
16. Barbato E, Carrié D, Dardas P, et al. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11:30-36.
17. De Maria GL, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc Interv. 2019;12:1465-1478.
18. Bittl JA, Chew DP, Topol EJ, Kong DF, Califf RM. Meta-analysis of randomized trials of percutaneous transluminal coronary angioplasty versus atherectomy, cutting balloon atherotomy, or laser angioplasty. J Am Coll Cardiol. 2004;43:936-942.
19. Safian RD, Feldman T, Muller DW, et al. Coronary angioplasty and Rotablator atherectomy trial (CARAT): immediate and late results of a prospective multicenter randomized trial. Catheter Cardiovasc Interv. 2001;53:213-220.
20. Abdel-Wahab M, Richardt G, Joachim Büttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6:10-19.
21. Abdel-Wahab M, Toelg R, Byrne RA, et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ Cardiovasc Interv. 2018;11:e007415.
22. Iannaccone M, Piazza F, Boccuzzi GG, et al. ROTational AThErectomy in acute coronary syndrome: early and midterm outcomes from a multicentre registry. EuroIntervention. 2016;12:1457-1464.
23. Kawamoto H, Latib A, Ruparelia N, et al. In-hospital and midterm clinical outcomes of rotational atherectomy followed by stent implantation: the ROTATE multicentre registry. EuroIntervention. 2016;12:1448-1456.
24. de Waha S, Allali A, Büttner HJ, et al. Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: Two-year clinical outcome of the randomized ROTAXUS trial. Catheter Cardiovasc Interv. 2016;87:691-700.
25. Cardiovascular System (CSI). Diamondback 360 Coronary Orbital Atherectomy System. Disponible en: https://csi360.com/diamondback-coronary-orbital-atherectomy-system/. Accessed 27 sep 2022.
26. Shlofmitz E, Shlofmitz R, Lee MS. Orbital Atherectomy: A Comprehensive Review. Interv Cardiol Clin. 2019;8:161-171.
27. Yamamoto MH, Maehara A, Karimi Galougahi K, et al. Mechanisms of Orbital Versus Rotational Atherectomy Plaque Modification in Severely Calcified Lesions Assessed by Optical Coherence Tomography. JACC Cardiovasc Interv. 2017;10:2584-2586.
28. Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510-518.
29. Redfors B, Sharma SK, Saito S, et al. Novel Micro Crown Orbital Atherectomy for Severe Lesion Calcification: Coronary Orbital Atherectomy System Study (COAST). Circ Cardiovasc Interv. 2020;13:e008993.
30. Parikh K, Chandra P, Choksi N, Khanna P, Chambers J. Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions: the ORBIT I trial. Catheter Cardiovasc Interv. 2013;81:1134-1139.
31. Lee M, Généreux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18:261-264.
32. Tsutsui RS, Sammour Y, Kalra A, et al. Excimer Laser Atherectomy in Percutaneous Coronary Intervention: A Contemporary Review. Cardiovasc Revasc Med. 2021;25:75-85.
33. Golino L, Caiazzo G, Calabrò P, et al. Excimer laser technology in percutaneous coronary interventions: Cardiovascular laser society’s position paper. Int J Cardiol. 2022;350:19-26.
34. Ojeda S, Azzalini L, Suárez de Lezo J, et al. Excimer laser coronary atherectomy for uncrossable coronary lesions. A multicenter registry. Catheter Cardiovasc Interv. 2021;98:1241-1249.
35. Mintz GS, Kovach JA, Javier SP, et al. Mechanisms of lumen enlargement after excimer laser coronary angioplasty. An intravascular ultrasound study. Circulation. 1995;92:3408-3414.
36. Fernandez JP, Hobson AR, McKenzie D, et al. Beyond the balloon: excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and non-expansible coronary lesions. EuroIntervention. 2013;9:243-250.
37. Latib A, Takagi K, Chizzola G, et al. Excimer Laser LEsion modification to expand non-dilatable stents: the ELLEMENT registry. Cardiovasc Revasc Med. 2014;15:8-12.
38. Sintek M, Coverstone E, Bach R, et al. Excimer Laser Coronary Angioplasty in Coronary Lesions: Use and Safety From the NCDR/CATH PCI Registry. Circ Cardiovasc Interv. 2021. https://doi.org/10.1161/CIRCINTERVENTIONS.120.010061.
39. Protty MB, Hussain HI, Gallagher S, et al. Excimer laser coronary atherectomy during complex PCI: An analysis of 1,471 laser cases from the British Cardiovascular Intervention Society database. Catheter Cardiovasc Interv. 2021;97:E653-E660.
40. Unterberg C, Buchwald AB, Barath P, Schmidt T, Kreuzer H, Wiegand V. Cutting balloon coronary angioplasty -- initial clinical experience. Clin Cardiol. 1993;16:660-664.
41. Barath P, Fishbein MC, Vari S, Forrester JS. Cutting balloon: a novel approach to percutaneous angioplasty. Am J Cardiol. 1991;68:1249-1252.
42. Bonaventura K, Schwefer M, Yusof AKM, et al. Systematic Scoring Balloon Lesion Preparation for Drug-Coated Balloon Angioplasty in Clinical Routine: Results of the PASSWORD Observational Study. Adv Ther. 2020;37:2210-2223.
43. Okura H, Hayase M, Shimodozono S, et al. Mechanisms of acute lumen gain following cutting balloon angioplasty in calcified and noncalcified lesions: an intravascular ultrasound study. Catheter Cardiovasc Interv. 2002;57:429-436.
44. Barbato E, Shlofmitz E, Milkas A, Shlofmitz R, Azzalini L, Colombo A. State of the art: evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses - from debulking to plaque modification, a 40-year-long journey. EuroIntervention. 2017;13:696-705.
45. de Ribamar Costa JJ, Mintz GS, Carlier SG, et al. Nonrandomized comparison of coronary stenting under intravascular ultrasound guidance of direct stenting without predilation versus conventional predilation with a semi-compliant balloon versus predilation with a new scoring balloon. Am J Cardiol. 2007;100:812-817.
46. Felekos I, Karamasis GV, Pavlidis AN. When everything else fails: High-pressure balloon for undilatable lesions. Cardiovasc Revasc Med. 2018;19:306-313.
47. Díaz JF, Gómez-Menchero A, Cardenal R, Sánchez-González C, Sanghvi A. Extremely high-pressure dilation with a new noncompliant balloon. Tex Heart Inst J. 2012;39:635-638.
48. Secco GG, Ghione M, Mattesini A, et al. Very high-pressure dilatation for undilatable coronary lesions: indications and results with a new dedicated balloon. EuroIntervention. 2016;12:359-365.
49. Secco GG, Buettner A, Parisi R, et al. Clinical Experience with Very High-Pressure Dilatation for Resistant Coronary Lesions. Cardiovasc Revasc Med. 2019;20:1083-1087.
50. Rheude T, Rai H, Richardt G, et al. Super high-pressure balloon versus scoring balloon to prepare severely calcified coronary lesions: the ISAR-CALC randomised trial. EuroIntervention. 2021;17:481-488.
51. Delgado-Arana JR, Rumoroso JR, Regueiro A, et al. Plaque modification in calcified chronic total occlusions: the PLACCTON study. Rev Esp Cardiol. 2022;75:213-222.
52. Vilalta del Olmo V, Rodríguez-Leor O, Redondo A, et al. Intracoronary lithotripsy in a high-risk real-world population. First experience in severely calcified, complex coronary lesions. REC Interv Cardiol. 2020;2:76-81.
53. Hill JM, Kereiakes DJ, Shlofmitz RA, et al. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J Am Coll Cardiol. 2020;76:2635-2646.
54. Kereiakes DJ, Hill JM, Shlofmitz RA, et al. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Lesions: 1-Year Results From the Disrupt CAD III Study. J Soc Cardiovasc Angiogr Interv. 2022. https://doi.org/10.1016/j.jscai.2021.100001.
55. Ali ZA, Nef H, Escaned J, et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses: The Disrupt CAD II Study. Circ Cardiovasc Interv. 2019;12:e008434.
56. Pham V, Bonnet M, Varenne O, et al. In-stent use of Intravascular Coronary Lithotripsy for restenosis and stent underexpansion, a multicenter experience. Can J Cardiol. 2022;10:1474-1475.
57. Achim A, Alampi C, Krivoshei L, Leibundgut G. In vitro effect of intravascular lithotripsy on the polymer of a drug-eluting stent. EuroIntervention. 2022;18:e333-e334.
58. Tovar Forero MN, Sardella G, Salvi N, et al. Coronary lithotripsy for the treatment of underexpanded stents; the international & multicentre CRUNCH registry. EuroIntervention. 2022;18:574-581.
59. Gonzálvez-García A, Jiménez-Valero S, Galeote G, Moreno R, López de Sá E, Jurado-Román A. “RotaTripsy”: Combination of Rotational Atherectomy and Intravascular Lithotripsy in Heavily Calcified Coronary Lesions: A Case Series. Cardiovasc Revasc Med. 2022;35:179-184.
60. Yarusi BB, Jagadeesan VS, Hussain S, et al. Combined Coronary Orbital Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Coronary Stenoses: The First Case Series. J Invasive Cardiol. 2022;34:E210-E217.
61. Jurado-Román A, García A, Moreno R. ELCA-Tripsy: Combination of Laser and Lithotripsy for Severely Calcified Lesions. J Invasive Cardiol. 2021;33:E754-E755.
62. Amemiya K, Yamamoto MH, Maehara A, et al. Effect of cutting balloon after rotational atherectomy in severely calcified coronary artery lesions as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 2019;94:936-944.
63. Jurado-Román A, Gonzálvez A, Galeote G, Jiménez-Valero S, Moreno R. RotaTripsy: Combination of Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Lesions. JACC Cardiovasc Interv. 2019;12:e127-e129.
64. Chen G, Zrenner B, Pyxaras SA. Combined Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified in-Stent Neoatherosclerosis: A Mini-Review. Cardiovasc Revasc Med. 2019;20:819-821.
65. Protty MB, Gallagher S, Farooq V, et al. Combined use of rotational and excimer lASER coronary atherectomy (RASER) during complex coronary angioplasty - An analysis of cases (2006-2016) from the British Cardiovascular Intervention Society database. Catheter Cardiovasc Interv. 2021;97:E911-E918.
66. McInerney A, Escaned J, Gonzalo N. Calcified coronary artery disease: pathophysiology, intracoronary imaging assessment, and plaque modification techniques. REC Interv Cardiol. 2022;4:216-227.
67. Bulluck H, McEntegart M. Contemporary tools and devices for coronary calcium modification. JRSM Cardiovasc Dis. 2022. https://doi.org/10.1177/20480040221089760.
68. Ambrosini V, Sorropago G, Laurenzano E, et al. Early outcome of high energy Laser (Excimer) facilitated coronary angioplasty ON hARD and complex calcified and balloOn-resistant coronary lesions: LEONARDO Study. Cardiovasc Revasc Med. 2015;16:141-146.
69. Karacsonyi J, Armstrong EJ, Truong HTD, et al. Contemporary Use of Laser During Percutaneous Coronary Interventions: Insights from the Laser Veterans Affairs (LAVA) Multicenter Registry. J Invasive Cardiol. 2018;30:195-201.
70. Yamamoto MH, Maehara A, Kim SS, et al. Effect of orbital atherectomy in calcified coronary artery lesions as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 2019;93:1211-1218.
* Corresponding author.
E-mail address: alfonsojuradoroman@gmail.com (A. Jurado-Román).
ABSTRACT
Bayesian statistics assesses probabilistically all sources of uncertainty involved in a statistical study and uses Bayes’ theorem to sequentially update the information generated in the different phases of the study. The characteristics of Bayesian inference make it particularly useful for the treatment of cardiological data from experimental or observational studies including different sources of variability, and complexity. This paper presents the basic concepts of Bayesian statistics associated with the estimation of parameters and derived quantities, new data prediction, and hypothesis testing. The latter in the context of model or theory selection.
Keywords: Posterior distribution. Prior distribution. Predictive distribution. Bayesian probability. Bayes’ theorem.
RESUMEN
La estadística bayesiana valora de forma probabilística cualquier fuente de incertidumbre asociada a un estudio estadístico y utiliza el teorema de Bayes para actualizar, de manera secuencial, la información generada en las diferentes fases del estudio. Las características de la inferencia bayesiana la hacen especialmente útil para el tratamiento de datos cardiológicos procedentes de estudios experimentales u observacionales que contienen diferentes fuentes de variabilidad y complejidad. En este trabajo se presentan los conceptos básicos de la estadística bayesiana relativos a la estimación de parámetros y cantidades derivadas, predicción de nuevos datos y contrastes de hipótesis; estos últimos en el contexto de la selección de modelos o teorías.
Palabras clave: Distribución a posteriori. Distribución previa. Distribución predictiva. Probabilidad bayesiana. Teorema de Bayes.
INTRODUCTION: MATHETMATICS, PROBABILITY, AND STATISTICS
In the words of world famous astrophysicist Stephen Hawking, the goal of science is «nothing less than a complete description of the universe we live in».1 Male and female scientists alike pursue this objective by building theories and assessing their predictions. It is the very essence of the scientific method.
Statistics is a scientific discipline that designs experiments and learns from data. It formalizes the process of learning through observations and guides the use the knowledge accumulated in decision-making processes. Concepts like chance, uncertainty, and luck are almost as old as mankind, and reducing uncertainty has always been a common goal for most human civilizations. Probability is the mathematical language used to quantify uncertainty and is at the core of statistical learning that represents—in probabilistic terms—both the study populations and the random samples that come from such populations.
There is not such a thing as a single statistical methodology. The most widely known and used ones are, by far, frequentist statistics, and Bayesian statistics. Both share common goals and use probability as the language of statistical learning. However, both understand the concept probability different. As a matter of fact, it is the element on which they largely disagree. According to the frequentist concept, it is only legitimate to assign probabilities to random phenomena that can be defined through experiments that can be repeated multiple times, and only under identical and independent conditions.
The Bayesian concept of probability is a much wider idea because it allows us to assign probabilities to all elements with uncertainty regardless of their nature. Bayesian probability applies to the occurrence of random events, both those that can be repeated under the conditions required by frequentist probability and those that don’t (chances that Arnau, a 60-year-old male who lives alone will recover from a heart attack). The differences between both methodologies grow even larger because Bayesian probability assigns probabilities to different parameters (like the prevalence of people between the ages of 45 and 65 who have suffered a heart attack), statistical hypotheses (the efficacy of a new treatment for diabetic patients with heart failure is greater compared to conventional treatment), probabilistic models or even to missing data generated by non-randomized losses to follow-up (eg, ignoring the information of patients with losses to follow-up in a survival study on a given end-stage process would introduce biased information to the study).
The second distinctive element between both statistical methodologies is the use of Bayes’ theorem. For Bayesian statistics it is an essential tool to sequentially update the relevant information that comes from a study. Therefore, after an initial analytical phase, the knowledge generated will be used to start a new process of learning that will be providing new information on the problem at stake.
Both the frequentist and Bayesian concepts of probability share the same axiomatic system, and the same probabilistic properties. This common niche makes them share a common mathematical language too.
The map of basic Bayesian concepts and their different associations is not easy to explain without falling into a plethora of technicalities. And this is even more evident in real-world studies in the cardiovascular research setting. Therefore, in this article we will be working on very clear cases we believe are powerful examples regarding conceptual terms that are, nonetheless, simple, and devoid of technical complexities.
This article includes 6 different sections. The first one, this introduction, refers to the general wisdom regarding Bayesian statistics and its association with mathematics, probability, and statistics. The second section includes brief historical references on Bayesian methodology. The next section is about Bayes’ theorem in its most innocent version regarding the occurrence of random events. Afterwards, we’ll be dealing with the concepts and basic protocol of Bayesian statistics: previous distribution, function of verisimilitude, posterior distribution, and predictive distribution to predict experimental results. Also, we’ll include a brief explanation on the computational problems associated with the practical application of Bayesian methods and their power to generate inferences on relevant derived quantities. Hypothesis testing—the P value in particular—will also be dealt with later on as well as the Bayesian hypothesis testing proposal. The article will end with a small comment on the use of prior distributions.
IT ALL STARTED WITH BAYES, PRICE, AND LAPLACE
Knowing a little bit of Bayesian history is important because it allows us to put it into a temporal and social perspective that illuminates and boosts its learning. We’ll give a few relevant hints on this history now. McGrayne2 gives us an easy-to-understand and rigorous big picture on Bayesian history.
The very first time anybody heard of Bayes’ theorem was in Great Britain halfway into the 18th century through Reverend Thomas Bayes while trying to prove the existence of God through mathematics. He would never dare to publish his findings. Prior to his death, he bequeathed all his savings to his friend Richard Price who—if okay with it—was supposed to spend this money to publish the findings, which is something he eventually did. However, these results went totally unnoticed.
We’re still in the 18th century, but now we’ll have to travel to France to meet Pierre-Simon Laplace, one of the most prominent mathematicians in history. He discovered, independently of Bayes and Price, Bayes’ theorem in the format that we know today. Also, he developed the Bayesian concept of probability. After his death, his work fell into oblivion, under attack too because it was not in tune with the ruling idea of objectivity so embedded in the scientific world at the time.
Back to Great Britain now. Bletchley Park was a 19th century mansion in Northern London turned into a working center to break the secret messages of the German army during the Second World War (1939-1945). Here Alan Turing and his team—that included the Bayesian statistician Jack Godd—played a key role in the history of Bayesian statistics: Bayes’ theorem was tremendously useful to decipher the code of the Enigma machines the Germans were using to code and decode messages. After the war, the British government classified all the information that had anything to do with Turing, mathematics, statistics, and decoding as top secret. Bayes’ theorem became a useful tool for just a handful of scientists, and an anathema (or worse) for most of them. As a truly revealing anecdote, McGrayne2 tells the story when Good presented the details of the method that Turing and his team had used to decipher the Nazi codes to members of Britain’s Royal Statistical Society. This is what the next speaker had to say about the whole thing: «After that nonsense […]».
During the second half of the 20th century, the future of Bayesian statistics looked grim: support from the English-speaking academic world grew thin, and the rest of the scientific community knew very little about Bayesian statistics. Also, there were many computational difficulties to implement it to real-world studies with data. But what seemed to be destined to happened never did. We’re now back to the Second World War to Los Alamos National Laboratory in the state of New Mexico, United States. This center was created with absolute secrecy during Second World War to investigate the construction of nuclear weapons under the umbrella of the so-called Manhattan Project, led by the United States with the participation of Great Britain and Canada. It is in this context where the early Monte Carlo simulation methods were discovered back in 1946 by Polish mathematician Stanislaw Ulam while playing solitaire. Also, at that time, Metropolis et al.3 publish the first Markov chain Monte Carlo (MCMC) simulation algorithm while conducting his investigations on the H-bomb.
Several years go by without any direct links whatsoever between Bayesian statistics and MCMC methods. However, some studies are published—especially on image recognition—combining both elements.4 Encouraged by the technological advances made, especially in computing, Alan Gelfand, an American, and Adrian Smith, a British, collect former studies on MCMC methods and make a direct connection with Bayesian statistics.5 This will mark the beginning of the great Bayesian revolution that starts in the field of applications to, little by little, move on to the academic world. Bayesian inference is now recognized, accepted, and validated by the scientific community as a useful statistical methodology for scientific and social development.
BAYES’ THEOREM
The most widely known format of Bayes’ theorem is presented for the occurrence of random events. If A and B are random events, then
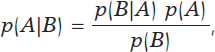
being p(A) the probability of event A, p(A|B) the associated probability A conditioned by the information that B occurred, and comparably p(B)> 0 and p(B|A). It is important to distinguish between probabilities p(A) and p(A|B). Both quantify the occurrence of A, but p(A) does so in absolute terms while p(B|A) does so in relative terms and conditioned by the information picked up in B. For example, nobody would question that the chances that a person may suffer from angina pectoris are higher if this person is hypertensive as opposed to not having that information available, p (Angina pectoris|Hypertension) > p (Angina pectoris).
Example I: Infections and tests
The prevalence that a certain infection affects any given population is .004. There is a test to detect its presence with a 94% sensitivity and a 97% specificity. We want to assess the chances that a person from such population is really infected if he tested positive for an infection.
We will use V and Vc to define a success that describes whether a person is infected or not, respectively. Therefore, p(V) = .004 and p(Vc) = .996. We’ll use (+) and (–) to describe a positive and negative test result for infection, respectively. In probabilistic terms, if a person is infected, he will test positive with a .94 probability, and negative with a .06 probability, p(+|V) = .94 and p(–|V) = .06 (false negative). If not infected, the person will test negative with a .97 probability and positive with a .03 probability, p(–|Vc) = .97 and p(+|Vc) = .03 (false positive).
According to Bayes’ theorem, the chances that a person is infected with a positive test result are
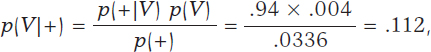
being p(+) = p(+|V) p(V) + p(+|Vc) p(Vc) = .0336 after implementing the total probability theorem (figure 1).
Figure 1. Posterior probability of infection with a positive test result p(V|+) (upper chart) and a negative test result p(V|–) (upper chart) in relation to the prior probability, p(V), of infection.
In principle, it is disconcerting that such a reliable test and with a positive test result for infection generates a small posterior probability, .112 favorable to the infection. However, if we take into consideration that the initial probability of being infected is p(V) = .004 and that, after the positive test result, probability is p(V|+) = .112, we’ll see that it has gone up from 4 to 112 by a thousand—has multiplied by a factor of 28—and we believe that the influence of the test result in such posterior probability is more relevant. Anyways, we would, at least, need a second test to increase the evidence for or against the infection.
Figure 2 shows 2 charts. The upper curve is the posterior probability of infection when the test is positive, p(V|+). The lower curve is the probability of infection too, but with a negative test result, p(V|–). In both cases, such posterior probabilities are represented in terms of prior probability, p(V), of being infected. When p(V) is close to 0, as it is the case with this example, the probability p(V|+) goes up a lot although, in absolute terms, it remains very low. On the contrary, when p(V) is close to 1, the probability p(V|–) will still be high despite evidence against a very reliable negative test result. The main element to understand this situation is that posterior probability, p(V|+) = .112, combines a very small probability of having an infection with a very high probability of testing positive when infected.
Figure 2. Approximate number of people infected, not infected, true positives, true negatives, false positives, and false negatives in a population of 100 000 inhabitants with an early prevalence for infection of .004 and a 94% sensitivity test and a 97% specificity test.
We’ll move on now to assess our results. The prevalence of infection, p(V) = .004 indicates that in a population of 100 000 people we should be expecting around 400 people infected and, approximately, 99 600 people not infected (figure 2). If the entire population were tested, we would expect to see that around 376 of the 400 people infected would test positive (true positives), as opposed to 24 (false negatives). In the group of healthy people, around 96 612 people would test negative (true negatives), but nearly 2988 people would test positive (false positives). If we looked at the number of people who tested positive, we’d have around 376 true positives, and 2988 false positives. Therefore, most people with a positive test (nearly 89%) would not actually be infected.
A second test with a positive result too would provide further evidence favorable to the infection. Its probability should be updated including the positive result of the second test as new information. If now (+1) and (+2) represent a positive test result for the first and second tests, the relevant probability would be p(V|+1,+2). The sequential use of Bayes’ theorem allows us to estimate such probability considering p(V|+1) = .112 as prior probability. The result obtained, p(V|+1,+2) = .798, is meaningful evidence favorable to an infection after 2 positive test results.
PARAMETER ESTIMATE
The true protagonists of basic Bayesian studies are probabilistic models governed by unknown parameters. These are at the core of Bayesian inferential machinery. We should mention that a parameter is a characteristic of a statistical population under study. Examples of parameters are the percentage of effectiveness of any given drug, the 5-year survival rate of soft tissue sarcoma, the basic reproduction factor R0 of an infection, etc. Parameters are estimated using partial information of the study population from data samples obtained through random procedures that guarantee their representativity and small population condition.
The most widely known probabilistic model is normal distribution with a dome-shaped symmetrical density function and defined by 2 different parameters, mean, µ, and standard deviation (σ). Mean is the center of gravity of distribution and corresponds to the peak of the dome. Standard deviation is a dispersion measurement that determines the width of the dome: in all normal distributions, the interval (µ − 3σ, µ + 3σ) includes 99.7% of the values of distribution. Therefore, the interval-related probability (−3, 3) in a normal distribution with mean = 0 and standard deviation = 1 would be the same as the one associated with the interval (−6, +6) of a normal distribution with mean = 0 and standard deviation = 2 (figure 3).
Figure 3. Chart of normal density with mean of 0, and standard deviation of 1 (green color), and normal density with mean of 0, and standard deviation of 2 (gray color).
Mean and standard deviation are unknown parameters in most studies based on normal data. In our case, and to avoid any technical complications, we’ll assume that the standard deviation is known. Therefore, the statistical process will only have eyes from the mean µ. Bayes’ theorem adapts itself to the territory of probability distributions with focus on the population mean symbol µ as parameter of interest according to the following formula
being p(μ) the previous distribution (or prior distribution) of μ that quantifies, in probabilistic terms, the initial information available on μ and p(μ | data), the posterior distribution of µ that contains the information on µ available when the initial information is added to data. The term p(data | μ) is the verisimilitude function of μ, a measurement that assesses the compatibility of data with the possible μ values. The element p(data) is the previous predictive distribution (also evidence in the automatic and machine learning setting), and assesses the plausibility of the data obtained.
Example II: the heart of boys and girls with spinal muscular atrophy
Falsaperla et al.6 present the results of an observational study on the heart electrical conduction system disorder that causes bradycardia or electrocardiographic abnormalities in boys and girls with spinal muscular atrophy type 1 and 2 (SMA1, and SMA2, respectively). We gained our inspiration from this study to build a simulated database. Therefore, the results from this example do not come from Falsaperla et al.6 and should not be compared to those from the original study.
We simulated the data on the PR interval length that extends from the origin of atrial depolarization until the origin of ventricular depolarization from 14 children with SMA2. We assumed a normal model with unknown measurement and known standard deviation. Out statistical goal was to estimate the mean.
We’ll go on now with the Bayesian protocol. First, we need a previous distribution p(μ) to express our information of such parameter. Afterwards, we consider a scenario without new information on μ except for the information provided by data and use Jeffreys Prior to treat all possible μ values the same way.7 The posterior distribution of μ, p(μ | data), is a normal distribution with a mean of .13, and a standard deviation of .03/√∙14 seconds that we can graphically see on figure 4. We estimate that μ is .13 seconds, and directly assess the accuracy of such estimate through a credibility interval that tells us that the posterior probability of μ will be taking values between .114 and .146 seconds is .95. We give it a very low probability of .05 that μ will be > .146 or < .114.
Figure 4. Posterior distribution of the PR interval mean length in children with spinal muscular atrophy type 2.
A frequentist analysis of this data would never allow direct probabilistic assessments of μ. A frequentist 95% confidence interval for μ would provide the same numerical results compared to the Bayesian interval. However, it should be interpreted in a completely different way. The frequentist 95% confidence interval is on the capacity of the interval to include μ true value, and not on the possible μ values. The interval built, (.114, .146), has a .95 probability of capturing μ true value, but also a .05 probability of not doing so. We should remember that the frequentist concept of probability prevents allocating probabilities to parameters and establishing direct probabilistic assessments of μ.
PREDICTING NEW OBSERVATIONS
Prediction and estimation are fundamental statistical concepts. We estimate parameters, but we predict data and experimental results always through distributions of probability.
The posterior predictive distribution of the results of a future experiment is built by combining the probabilistic model that correlates future data and parameters with posterior distribution. A significant aspect of predictive process regarding estimation is its greater uncertainty. Overall, the precision of estimations improves with larger samples, which means that in the hypothetical case of having all the data available, our estimation would be accurate. The process of prediction does not have such a feature. Although the precision of predictions increases parallel to the size of the sample, in the hypothetical case of having access to all the information from a population, error-free predictions would still be impossible.
Example II: the heart of boys and girls with muscular atrophy (continues)
In the estimation stage we studied the PR interval mean length in girls and boys with SMA2, learning based on a sample of 14 simulated data. Now we’ll be dealing with totally different situation. We have a kid with SMA2 who did not participate in the study. Our objective is to predict his PR interval length. The goal now is not to estimate population means with SMA2, but to predict the value of the PR interval length in a particular kid.
Figure 5 shows the posterior predictive distribution of the PR interval length of a new boy with SMA2. Although this prediction is based on information from 14 children from the sample, it is about a new boy with SMA2. The anticipated value of this new child’s PR interval length is .13 seconds. The accuracy of prediction is quantified through prediction intervals. In this case with a .95 probability, the anticipated value will be between .069 and .191 seconds.
Figure 5. Posterior predictive distribution of the PR interval length of a new child with spinal muscular atrophy type 2.
SIMULATION AND GROUP COMPARISON
The Bayesian protocol with the 3 basic elements, previous distribution, verisimilitude function, and posterior distribution is common to almost all kinds of settings, both the basic ones including some parameters, as well as the complex ones with many sources of uncertainty with complex hierarchical structures. It is a robust and easy-to-use protocol, and a powerful and appealing idea.
Difficulties appear if we want to extrapolate this protocol to real studies of certain complexity. It is in just a couple of these cases that an analytical expression for the posterior distribution of parameters can be achieved. Impossible, nonetheless, for posterior distributions associated with derived quantities of interest. In most studies, mathematics becomes complicated, and posterior distributions are difficult to obtain. In these cases, the MCMC methods come to the rescue of Bayesian analysis. They can simulate our estimates of the relevant posterior distribution and from them generate the inferences or predictions required by the study.
In the following example we’ll be showing the most basic situation we have discussed: starting with a target posterior (analytical) distribution we’ll be simulating posterior distributions of relevant, non-analytical quantities of interest.
Example III: acute myocardial infarction and stents
The following example has been inspired by Iglesias et al.8. This is a study of 1300 patients with acute myocardial infarction treated with percutaneous coronary intervention. Each patient was randomized to sirolimus-eluting stent implantation with degradable polymer (group S) or everolimus-eluting stent implantation with durable polymer (group E).
We compared both treatments in relation to the rate of deaths 12 months after treatment. A total of 35 out of the 649 patients from group S stopped treatment or were lost within first year of follow-up, and 24 died. In group E, initially with 651 patients, 25 were lost or stopped treatment, and 22 died. The presence of missing data due to losses to follow-up is an important issue that should be dealt with carefully. In this case we’ll omit it because our goal is to illustrate Bayesian procedures using the least possible technicalities.
We’ll start by analyzing the risk of death θs and θE in groups S and E, respectively 1 year into treatment. Since anybody from either one of the 2 groups can die, or not, within the first year of treatment, in each group, the probabilistic model is binomial distribution that will be describing the number of deaths reported. The risk of death from each group is a rate with values that range between 0 and 1. For each rate we’ll be selecting beta distribution because it’s the proper probabilistic model to use with rates and doesn’t pose any estimate difficulties. β distribution (that we’ll representi as Be(α, β)) has 2 different parameters, α > 0 and β > 0, that determine the way of the distribution as well as its mean and variance. It is a flexible distribution that can be symmetrical or asymmetrical, positive or negative (figure 6).
Figure 6. Chart of β densities: Be(.5, .5) in green color, Be(2, 2) in gray color, Be(3, 22) in maroon color, Be(12, 2) in blue color, and Be(12, 12) in pumpkin color.
The prior β distribution that best describes the lack of information is Be(.5, .5). Its justification only responds to theoretical criteria. In each group, the posterior distribution of the risk of death will also be β whose updated parameters can be obtained by adding the number of deaths and the number of people alive in the study to the 2 values 0.5 and 0.5 of the prior beta distribution:
p(θs) = Be(.5, .5); p(θs | data) = Be(24.5, 590.5),
p(θE) = Be(.5, .5); p(θE | data) = Be(22.5, 604.5).
The estimation of the risk of death in patients from groups S and E is the mean of its posterior distribution, .040 and .036, respectively. Also, with a .95 probability the risk of death of group S is between .026 and .057, and between .023 and .052 in group E. These results indicate that the rate of death from both groups is small, although slightly higher in group S. The 95% credibility interval—both of θs and θEis very informative (figure 7).
Figure 7. Posterior distribution of the mortality rate reported in patients from group S (green color) and group E (gray color).
We assume that our goal is to compare the 1-year mortality risk in both groups. Although the tool we could think of first is hypothesis testing (that we’ll introduce later on), the epidemiological and statistical literature on this regard is abundant, and 2 groups are often compared through relative risk (RR) or absolute risk (AR).9 The 1-year RR of mortality in patients with type S stents vs patients with type E stents is RR = θs/θE, a ratio between 2 rates. RR values < 1 are indicative that the mortality rate from group S is lower compared to group E while values > 1 are indicative of precisely the opposite. Since RR is defined through θs and θE, the information from both rates, expressed through its posterior distribution, can propagate to RR as a posterior probability distribution, p(RR | data) (figure 8). This distribution is not analytical, but it can come close to it through a Monte Carlo simulation from the 2 posterior distributions p(θs | data) and p(θE | data). Using this approximation, the posterior RR mean is 1.160, its standard deviation, .346, and the posterior probability of RR being >1 is .641. An analogue frequentist analysis would be more complicated from the mathematical standpoint and would not provide a direct probabilistic assessment of RR.
Figure 8. Posterior distribution of the relative risk of mortality at 1 year in patients with S-type stents vs patients with E-type stents. The shadow region is the probability, .641, that the mortality rate of group S is higher compared to group E.
If we compare both groups through the AR of mortality at 1 year, our goal would be AR = θs-θE. This is a difference between 2 rates that could take values between −1 and 1. Negative values will be indicative that the mortality rate of group E is higher compared to groups S while positive values will indicate just the opposite. figure 9 shows the AR posterior distribution.
Figure 9. Posterior distribution of the absolute risk of mortality at 1 year in patients with S-type stents vs patients with E-type stents. The shadow region is the probability, .641, that the mortality rate of group S is higher compared to group E.
The posterior AR mean is .004, its standard deviation, .011, and with a .641 probability, the AR will be > 0.
HYPOTHESIS TESTING: FREQUENTIST P VALUES
Hypothesis testing is the topic that generates the most irreconcilable differences between the Bayesian and the frequentist scientific communities because it is here where the consequences of their different concepts of probability become more evident. Testing hypothesis means testing new theories. Most of the latest theories have a quiet appearance among the scientific community, but little by little they start accumulating evidence in their favor until the sitting evidence is debunked.
The most widely known and used concept in frequentist statistics is the P value, as well as its .05 value that in some studies appears as the magical number used to reject or accept hypotheses or scientific theories. P value is another tool in the frequentist inference armamentarium that simply does not exist in the Bayesian one regarding the testing of 2 different hypotheses: the null hypothesis H0 (that often represents the sitting scientific theory), and the alternative hypothesis H1 (the new theory). P values are always associated with data because without the latter there are no P values. Under these conditions, P value is the probability that a certain theoretical summary of data will be equal to the one observed or more incompatible with the null hypothesis supposing that such hypothesis holds true. Such compatibility is often represented by the P = .05 threshold. P values ≥ .05 keep confidence in the null hypothesis; P values < .05, however, are favorable to the alternative one.
The excessive, and sometimes, inappropriate use of P values in scientific studies is still under discussion in the statistical community. It started in small scientific circles, but the use of the P value soon became a somehow «magical» element rather than a scientific tool. Back in 2014, the American Statistical Association, one of the world’s leading statistical societies, approached this issue and drafted a document that has become the go-to guideline on this topic.10 The following ones are some of the conclusions on significant P values for the management of biometrical data:
- They are a probabilistic measurement of the compatibility of data with the null hypothesis. Smaller P values are associated with more data incompatibility with such hypothesis.
- Do not assess the probability that a hypothesis will hold true or not.
- The conclusions of a study should not only be based on whether a given P value exceeds this or that threshold. The use of the expression «statistically significant» (P < .05) to establish conclusions distorts all scientific procedures.
- Do not measure the size of an effect or the significance of a given result. All small effects can produce small P values when the size of the sample or the accuracy of measurements is big, and all big effects can generate big P values with small samples or imprecise observations.
The P value has been given an unfair treatment because it has been attributed fantastical and surreal properties that have turned against it. Controversy has shattered the scientific debate and encouraged criticism in scientific disciplines that use data to generate knowledge. The huge interest in today’s scientific reproducibility topics owes volumes to this debate.11-15
ACCUMULATING EVIDENCE FOR THE PROBABILISTIC ASSESSMENT OF NEW THEORIES
The Bayesian concept of probability is the key element to put hypotheses and theories to the test because it allows us to assign direct probabilities to both hypotheses and theories, both prior, p(theory holds true) and posterior, p(theory holds true|data ).16
Frequentist statistics is based on hypothesis testing using p-type probabilities (data|theory holds true) while Bayesian statistics is based on p-type probabilities (theory holds true|data). The p-type(data|theory holds true) frequentist probability assumes that the theory tested holds true, and based on that assumption assesses the concordance of data with such hypothesis. The p-type(theory holds true|data) Bayesian probability probabilistically assesses the certainty of the theory being tested in association with the data obtained.
The fundamental tool of Bayesian statistics to choose between hypotheses
H0: theory #1 holds true,
H1: theory #2 holds true,
based on a dataset is Bayes factor,17 the ratio between the probabilities associated with data according to both theories. It can also be expressed as the ratio between posterior odds (p(theory #1 holds true|data )/p(theory #2 holds true|data)) favorable to the certainty of theory #1 compared to theory #2 and the corresponding prior odds (p(theory #1 holds true)/p(theory #2 holds true). Like this:
Bayes factor (B) holds evidence favorable to the certainty of theory #1 (compared to theory #2) provided by data: it turns prior probabilities into posterior probabilities. In logarithmic scale, log (B), the Bayes factor is also known as «weight of evidence», a term coined by Turing back in Bletchley Park during Second World War. Small Bayes factor values give little support to H0 vs H1; however, big Bayes factor values provide extensive support to H0.
Example I: Infections and tests (continues)
Let’s go back to the data from example I: Vallivana needs to be diagnosed on an infection with 2 positive test results. This problem can be faced as 2 hypotheses being tested:
H0: Vallibana has an infection
H1: Vallibana doesn’t have an infection
We assume that Vallibana does not have any particular characteristics that give her a probability of infection different from the remainder of the population. Therefore, we know that p(Vallibana has an infection) = .004, and p(Vallibana doesn’t have an infection) = .996. Vallibana’s prior odds favorrable to the infection compared to non-infection are:
Vallibana takes the test, and it turns out positive (+1). She decides to retake it and tests positive again (+2.). Vallibana’s posterior odds favorable to the infection compared to non-infection are:
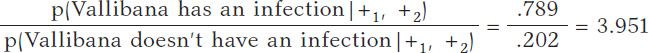
The Bayes factor in favor of Vallibana being infected, that is, the ratio between the posterior odds and the prior odds is 987.75. Indeed, this value provides strong evidence in favor of Vallibana being infected (+).
Example II: the heart of boys and girls with spinal muscular atrophy (continues)
Now let’s go back to the study conducted by Falsaperla et al.6 from example II that aimed to compare the PR interval mean length in girls and boys with SMA1 and SMA2—that we’ll refer to as μ1 and μ2, respectively—through hypothesis testing:
H0: μ2 ≤ μ1
H1: μ2 > μ1
where the null hypothesis, H0, claims that the PR interval mean length in girls and boys with SMA2 is shorter or equal to that reported in children with SMA1. The alternative hypothesis H1 says otherwise. Here we’ll be working with simulated normal data in both groups: n1 = 14 observations in the SMA1 group with sample mean and standard deviation values of .10 and .02 seconds, respectively, and n2 = 14 observations in the SMA2 group with sample mean and standard deviation values of.13 and .03 seconds, respectively.
What we´ll do is build an inferential process for the mean of each group separately. In both cases, we’ll be considering a neutral previous distribution that gives all prominence to data. figure 10 shows the posterior distribution of the mean of each group. Both distributions are rather separate from one another, which means that the posterior probabilities associated with each hypothesis will be very different as well: .002 for H0, and .998 for H1.
Figure 10. Posterior distribution of the PR interval mean length in girls and boys with spinal muscular atrophy type 1 (green color), and type 2 (gray color).
p(H0|data) = p(μ2 ≤ μ1|data) = .002
p(H1|data) = p(μ2 > μ1|data) = .998
On a roughly basis, it is 500 times more likely that H1 will hold true than not. In light of such an overwhelming piece of evidence the wise decision would be to choose H1. The frequentist treatment of this testing is based on the P value. In our case, we’d obtain a P value of .002, which would imply rejecting the null hypothesis in favor of the alternative one. Both methodologies propose the same decision and provide the same numerical results: probabilities of .002. However, both probabilities are conceptually different. Bayesian probability tests the null hypothesis based on the data reported. Frequentist probability assesses the data observed with the assumption that the null hypothesis will hold true.
Still following in the footsteps of Falsaperla et al.6 we wish to mention that our examples are not based on original cases. They are merely illustrative of Bayesian procedures. We’ll now be working with the P-wave on the electrocardiogram. We wish to compare the P-wave mean length in children with SMA1 and SMA2. We’ll be simulating 14 observations of the P-wave length in the group of children with SMA1 and compared it to children with SMAs. The sample mean and standard deviation is .09 and .05 seconds in group SMA1, respectively, and .07 and .03 seconds in group SMA2. We’ll be comparing the means of both groups through hypothesis testing:
H0: mean P-wave in SMA1 = mean P-wave in SMA2
H1: mean P-wave in SMA1 > mean P-wave in SMA2
based on Bayesian inferential process like the one from the previous example. figure 11 shows the posterior distribution of the P-wave mean length in both groups. There are fewer data from the group of girls and boys with SMA2 compared to the group with SMA1. The posterior probability associated with each hypothesis is:
p(H0 | data) = p(mean P-wave in SMA1 ≤ mean P-wave in SMA2 | data)
p(H1 | data) = p(mean P-wave in SMA1 > mean P-wave in SMA2 | data)
These results provide a significant piece of evidence favorable to the alternative hypothesis that is almost 8 times more likely than H0. From the frequentist standpoint, the P value associated with data would be .107 (>.05), which is why we would conclude that data does not provide enough evidence to reject H0. Bayesian decision could perfectly be the same. However, the Bayesian analysis provides a direct assessment on the certainty of both hypotheses. The findings from the Bayesian analysis could be used as previous information in future studies with more data. Therefore, the posterior distributions obtained (figure 11) would be prior distributions in this new study. Bayes’ theorem allows us to generate knowledge sequentially searching for evidence for or against different hypothesis.
Figure 11. Posterior distribution of the P-wave mean length in girls and boys with spinal muscular atrophy type 1 (green color), and type 2 (gray color).
CARDIOLOGY POSES SOME OF DOUBTS ON THE IMPLEMENTATION OF METHODOLOGY IN CLINICAL TRIALS
One of the most controversial topics in Bayesian methodology is the selection of previous distributions. A Bayesian analysis will always allow us to avoid using any information on the amount of interest not provided by data. In this case, it works with previous distributions that play a neutral role in the learning process and that are useful only as the starting point of the Bayesian inferential protocol.
Previous informative distributions contain information that adds to the one provided by data like expert knowledge18-20 or results from previous studies.21,22 It is a highly valuable Bayesian characteristic in studies on which data is scarce like studies on rare diseases and orphan drugs. We should mention that inferential processes based on previous informative distributions should include sensitivity analyses of the results obtained regarding previously used distribution or distributions. Similarly, it has become popular to consider communities of previous distributions with diverse previous distributions—and a certain degree of skepticism or enthusiasm—with the effect under test because they provide a scientific framework of reference.
On many occasions, in clinical trials, the use of previous informative distributions reduces the sizes of frequentist samples based on preassigned values of the test power, and previous parameter estimates.22 An example of this situation is the BIOSTEMI trial.8 The 1300 patient-sample was estimated using Bayesian methods through a previous robust distribution as a mixture that included—in equal proportion—historical information of 407 patients from the BIOSCIENCE trial,23 and a practically non-informative distribution. The flexibility of Bayesian sequential learning is a key element of the so-called adaptative Bayesian designs24 that allow us to include additional information in different phases of the trial without damaging the consistency and reliability of results.
FUNDING
This study was partially funded by Biotronik Spain S. A, and by project PID2019-106341GB-I00 from the Spanish Ministry of Science and Innovation, and Universities of the Spanish Government.
AUTHORS’ CONTRIBUTIONS
C. Armero was involved in the structure, content, and drafting of this manuscript; P. Rodríguez, and J.M. de la Torre Hernández were actively involved in the review process of the manuscript final version.
CONFLICTS OF INTEREST
C. Armero, P. Rodríguez, and José M. de la Torre Hernández declared no conflicts of interest regarding the content, authorship, and publication of this manuscript. J.M. de la Torre Hernández is the editor-in-chief of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
REFERENCES
1. Hawking S. A Brief History of Time: The Origin and Fate of the Universe. New York: Bantam; 1988.
2. McGrayne SB. La teoría que nunca murió: De cómo la regla de Bayes permitió descifrar el código Enigma, perseguir los submarinos rusos y emerger triunfante de dos siglos de controversia. Barcelona: Crítica; 2012.
3. Metropolis N, Rosenbluth A, Rosenbluth M, Teller A, Teller E. Equations of state calculations by fast computing machines. J Chem Phys. 1953;21:
1087-1092.
4. Robert CP, Casella G. A Short History of Markov Chain Monte Carlo: Subjective Recollections from Incomplete Data. Stat Sci. 2011;26:102-115.
5. Gelfand AE, Smith AFM. Sampling-based approaches to calculating marginal densities. J Am Stat Assoc. 1990;85:398-409.
6. Falsaperla R, Vitaliti G, Collotta AD, et al. Electrocardiographic Evaluation in Patients With Spinal Muscular Atrophy: A Case-Control Study. J Child Neurol. 2018;33:487-492.
7. Robert CP, Chopin N, Rousseau J. Harold Jeffreys’s Theory of Probability Revisited. Stat Sci. 2009;24:141-172.
8. Iglesias JF, Muller O, Heg D, et al. Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial. Lancet. 2019;394:1243-1253.
9. Christensen R, Johnson W, Branscum A, Hanson TE. Bayesian Ideas and Data Analysis: An Introduction for Scientists and Statisticians. Boca Raton: CRC Press; 2011.
10. Wasserstein RL, Lazar NA. The ASA Statement on p-Values: Context, Process, and Purpose. Am Stat. 2016;70:129-133.
11. Goodman SN. Toward Evidence-Based Medical Statistics. 1: The P Value Fallacy. Ann Intern Med. 1999;130:995-1004.
12. Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31:337-350.
13. Halsey LG, Curran-Everett D, Vowler SL, Drummond GB. The fickle P value generates irreproducible results. Nat Methods. 2015;12:179-185.
14. Ioannidis JPA. Why Most Published Research Findings Are False. PLoS Med. 2005;2:e124.
15. Ioannidis JPA. The Proposal to Lower P Value Thresholds to .005. J Am Med Assoc. 2018;319:1429-1430.
16. Kruschke JK. Doing Bayesian data analysis: A Tutorial with R, JAGS, and Stan. 2nd ed. Amsterdam: Academic Press/Elsevier; 2015.
17. Kass RE, Raftery AE. Bayes Factors. J Am Stat Assoc. 1995;90:773-795.
18. Hampson LV, Whitehead J, Eleftheriou D, et al. Elicitation of Expert Prior Opinion: Application to the MYPAN Trial in Childhood Polyarteritis Nodosa. PLoS One. 2015;10:e0120981.
19. Mason AJ, Gomes M, Grieve R, Ulug P, Powell JT, Carpenter J. Development of a practical approach to expert elicitation for randomised controlled trials with missing health outcomes: Application to the IMPROVE trial. Clin Trials. 2017;14:357-367.
20. Jansen JO, Wang H, Holcomb JB, et al. Elicitation of prior probability distributions for a proposed Bayesian randomized clinical trial of whole blood for trauma resuscitation. Transfusion. 2020;60:498-506.
21. Grant RL. The uptake of Bayesian methods in biomedical meta-analyses: A scoping review (2005–2016). J Evidence-Based Med. 2019;12:69-75.
22. Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. Chichester: Wiley; 2004.
23. Pilgrim T, Heg D, Roffi M, et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non- inferiority trial. Lancet. 2014;384:2111-2122.
24. Schmidli H, Gsteiger S, Roychoudhury A, O’Hagan A, Spiegelhalter D, Neuenschwander B. Robust meta-analytic-predictive priors in clinical trials with historical control information. Biometrics. 2014;70:1023-1032.
* Corrresponding author:
E-mail address: carmen.armero@uv.es (C. Armero).
ABSTRACT
Hypertension is the most prevalent cardiovascular risk factor. Despite pharmacological treatment, a high percentage of patients do not achieve an adequate blood pressure control. Renal sympathetic denervation is a minimally invasive intervention for the management of hypertension involving the interruption of the renal artery sympathetic nervous system using a catheter-based approach. The early studies showed promising results, but the controversial results coming from the SYMPLICITY HTN-3 trial sent this technique into oblivion. Over the last 3 years, new clinical trials have appeared including new devices used in different populations, which definitively proves the effectiveness of renal sympathetic denervation.
This joint position statement from the Spanish Society of Hypertension-Spanish League for Combating High Blood Pressure (SEH-LELHA), and the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) reviews the evidence available on the efficacy and safety profile of renal sympathetic denervation for the management of hypertension. Based on the results of clinical trials, recommendations have been established on what patients may be eligible for renal sympathetic denervation and under what circumstances.
Keywords: Hypertension. Renal sympathetic denervation. Blood pressure.
RESUMEN
La hipertensión arterial es el factor de riesgo cardiovascular más prevalente. A pesar del tratamiento farmacológico, un alto porcentaje de pacientes no consiguen un adecuado control. La denervación renal es una intervención mínimamente invasiva para el tratamiento de la hipertensión que implica la interrupción de los nervios simpáticos renales mediante un abordaje con catéter. Los estudios iniciales mostraron resultados prometedores, pero los controvertidos resultados del ensayo SYMPLICITY HTN-3 llevaron al abandono de la técnica. En los últimos 3 años han aparecido los resultados de nuevos ensayos clínicos, con nuevos dispositivos y en diferentes poblaciones, que demuestran definitivamente la eficacia de la denervación renal.
En este documento de posicionamiento conjunto de la Sociedad Española de Hipertensión-Liga Española para la Lucha contra la Hipertensión Arterial (SEH-LELHA) y la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (ACI-SEC) se revisa la evidencia disponible sobre la eficacia y la seguridad de la denervación renal en el tratamiento de la hipertensión. A partir de los resultados de los ensayos clínicos, se generan recomendaciones sobre qué pacientes y en qué condiciones podrían ser candidatos a una denervación renal.
Palabras clave: Hipertension arterial. Denervacion renal. Presion arterial.
Abbreviations ABPM: ambulatory blood pressure monitoring. BP: blood pressure. DBP: diastolic blood pressure. HMOD: hypertension-mediated organ damage. HTN: hypertension. RSD: renal sympathetic denervation. R-HTN: resistant hypertension. SBP: systolic blood pressure.
INTRODUCTION
The role of the sympathetic nervous system in the pathophysiology of hypertension (HTN) is well known. In 2007, to address the unmet need of patients with resistant HTN (R-HTN), the first percutaneous renal sympathetic denervation (RSD) procedures were performed. First observational studies showed positive results, and the use of RSD started in select centers around the world.1,2 However, in 2014, the publication of a study which did not demonstrate a greater efficacy of RSD vs a sham-control group to control blood pressure (BP)3 dramatically reduced the interest of the scientific community in this procedure, as well as in its clinical application. An increased knowledge of renal anatomy combined with the development of second-generation devices has led to new studies, in which the efficacy of RSD vs a sham-control group has been demonstrated.4-7 Although the road ahead is long, the new evidence provides a clear role for RSD in the management of patients with HTN.
The clinical practice guidelines on the management of hypertension published by the European Society of Cardiology and European Society of Hypertension (ESC/ESH) back in 2018 outlined the role of device-based approaches for the management of HTN in the context of clinical trials only.8 The practical effect of this is that it discouraged the use of RSD. Despite the short time that has gone by since the publication of these guidelines, the data provided by the new clinical trials would justify treating selected patients with RSD.
This document reviews the evidence available on RSD for the management of HTN, analyzes possible indications, and suggests strategies to identify potentially eligible patients, formulated from the opinion of a panel of experts selected by the Spanish Society of Arterial Hypertension-Spanish League for the fight against Arterial Hypertension (SEH-LELHA), and the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC). The writing of the document was carried out by professionals proposed by these scientific societies undersigning this work following their experience in the management of patients treated with RSD. After the first draft, other experts with and without previous experience in RSD carried out a critical review of the document, and agreed on the changes that were deemed appropriate.
CLINICAL EVIDENCE ON THE ROLE OF RENAL SYMPATHIC DENERVATION IN THE MANAGEMENT OF HYPERTENSION
The supplementary data shows the epidemiological characteristics of HTN (section 1), as well as the role of sympathetic nervous system in the management of HTN (section 2), thus improving our understanding of clinical trials. Table 1 of the supplementary data shows the main studies in which the efficacy of RSD has been assessed.
Table 1. Studies prior to renal sympathetic denervation in patients with uncontrolled hypertension
| Evaluation of pharmacological treatment |
| Type and number of drugs |
| Drug adequate dosage |
| Assess use of aldosterone antagonist |
| Assess lack of therapy compliance |
| Assess intolerance to drug therapy |
| 24-hour ABPM study |
| Rule out pseudo-resistant hypertension or white coat effect |
| Confirm uncontrolled hypertension (SBP > 130 mmHg/DBP > 80 mmHg at the 24-hour levels or SBP > 135/DBP > 85 in the day’s levels) |
| Rule out secondary causes of hypertension (table 2) |
| Cardiovascular risk assessment |
| Coexistence of other cardiovascular risk factors such as dyslipidemia, diabetes or smoking |
| Presence of HMOD |
| Presence of established cardiovascular or kidney disease |
| Imaging of the renal anatomy by computerized tomography or nuclear magnetic resonance imaging (assessment of occlusive stenosis, accessory branches, arterial diameter) |
| Complementary tests recommended: |
| Hemogram, renal function parameters, liver and lipid profiles, and urine sediment tests to detect the presence of microalbuminuria |
| Specific analytical determinations: |
| Baseline plasma aldosterone-to-renin ratio |
| Thyroid hormones |
| Calcium-phosphorus metabolism with parathyroid hormone levels |
| Cortisol (basal and 24-hour urine ratios) |
| Catecholamines with 24-hour urinary metanephrines ratio |
| Polysomnography |
ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure, HMOD, hypertension-mediated organ damage; SBP, systolic blood pressure. |
Back in 2009, the first study on RSD in patients with R-HTN, the SYMPLICITY HTN-1 trial, was published. This study suggested a high efficacy profile of RSD, and a lower office systolic blood pressure (SBP) down to 27 mmHg at 12 months, without any significant complications being reported.1
The SYMPLICITY HTN-3 trial was the first one to include a control group with a sham procedure and a 24-hour BP endpoint. No differences between the 2 treatment groups in terms of the efficacy profile of BP control in patients with R-HTN were reported at the 6-month follow-up.3 The disagreement between the results of this study and the previous ones, as well as the identification of several confounding factors9 brings up the need for designing new studies specifically aimed at solving these questions.
Definitive evidence on the efficacy of RSD has come from the SPYRAL HTN and RADIANCE-HTN studies. The SPYRAL HTN-ON MED trial enrolled patients with uncontrolled HTN treated with 1 to 3 antihypertensive drugs, randomized to receive RSD or a sham procedure. The 24-hour ambulatory SBP and diastolic BP (DBP) levels, as well as the office SBP and DBP levels dropped significantly in the RSD group compared to the sham control at the 6-month follow-up.4 With a similar design, the SPYRAL HTN-OFF MED Pivotal trial enrolled uncontrolled hypertensive patients with office SBP levels between 150 mmHg and 180 mmHg in the absence of antihypertensive treatment. The 24-hour SBP and office SBP levels were reported at the 3-month follow-up.5 The RADIANCE-HTN SOLO trial enrolled patients with HTN and ambulatory blood pressure monitoring (ABPM) levels ≥ 135/85 mmHg and ≤ 170/105 mmHg without pharmacological treatment. The 24-hour ambulatory SBP and DBP levels as well as the office SBP and DBP levels dropped significantly in the RSD group compared to the sham control at the 2-month follow-up.6 The RADIANCE-HTN TRIO trial enrolled patients with R-HTN on a fixed-dose, single-pill combination of a calcium channel blocker, an angiotensin receptor blocker, plus a thiazide diuretic, randomized to receive ultrasound catheter-based RSD or a sham procedure. The 24-hour ambulatory SBP and DBP levels, as well as office SBP and DBP levels dropped significantly in the RSD group compared to the sham control at the 2-month follow-up.7
Real-life registries have enrolled more than 3500 patients treated with RSD showing lower office BP and ABPM levels. Some registries have demonstrated that the reduction of BP is not associated with the medication burden or with an increased number of antihypertensive drugs. RSD has proven to be safe and has a low rate of complications associated with the procedure.10 The GLOBAL SYMPLICITY registry, with over 2900 patients is the largest and longest duration analysis to this date of renal sympathetic denervation to show the efficacy and safety profile of RSD in a real-life scenario.10 Table 2 of the supplementary data shows a summary of different registries on RSD.
Table 2. Causes of secondary hypertension
| Renal parenchymal diseases | Glomerulopathies |
| Renovascular diseases | Fibrodysplasia |
| Suprarrenal diseases | Primary hyperaldosteronism |
| Vasculares diseases | Aortic coarctation |
| Endocrine-metabolic | Thyroid dysfunction |
| Neurological diseases | Dysautonomiay |
| Toxic-pharmacological diseases | Corticosteroids |
| Genetic diseases | Monogenic forms |
RSD has been confirmed as a safe intervention. The incidence rate of both immediate complications associated with the procedure and renal and vascular complications in the short- and mid-term (6-12 months) is very low and is mainly associated with local problems at the puncture site; serious renal complications (renal artery dissection or stenosis) are anecdotal. Table 3 of the supplementary data summarizes the safety data from the main randomized clinical trials that often have a short-term clinical follow-up.
Table 3. Precautions and contraindications to renal sympathetic denervation
| • Renal sympathetic denervation has not been evaluated in patients who are pregnant, nursing, intend to become pregnant or in patients with type I diabetes mellitus, previous renal angioplasty, indwelling ureteral stents, aortic grafts or abnormal renal anatomy |
| • Subjects in whom a reduction of blood pressure would be considered hazardous (such as those with hemodynamically significant valvular heart disease) |
| • Implantable pacemakers and implantable cardioverter/defibrillators may be adversely affected by radiofrequency ablation. Consider deactivating implantable cardioverter/defibrillators during ablation, have temporary external sources |
| of pacing and defibrillation available during ablation, and perform a complete analysis of the functionality of the device implanted after ablation |
| • Avoid treating arteries with diameters < 3 mm or > 8 mm |
| • Avoid treating arteries with significant disease or flow-limiting obstructions |
POSSIBLE INDICATIONS FOR RENAL SYMPATHETIC DENERVATION WITH DATA FROM THE LATEST CLINICAL TRIALS
Data from both randomized clinical trials and registries prove that the RSD procedure is safe and effective reducing BP, which is consistent across different populations including high-risk subgroups, and with different devices. Section 3 of the supplementary data reviews various consensus documents and recommendations previously published by different scientific societies prior to the publication of the SPYRAL HTN and RANDIANCE-HTN clinical trials.
RSD can be considered in patients with resistant HTN (BP > 140/90 mmHg despite lifestyle changes treated with ≥ 3 antihypertensive drugs at optimal doses, one of them being a diuretic or HTN controlled with ≥ 4 drugs),8 and also in patients with uncontrolled HTN (BP > 140/90 mmHg in patients with poor therapeutic compliance), and high cardiovascular risk.
Renal sympathetic denervation in patients with resistant hypertension
Patients with R-HTN were the first group in whom the role of RSD was assessed. The SYMPLICITY HTN-3 trial failed to demonstrate the increased efficacy of RSD vs sham control in patients with R-HTN.3 However, subsequent analysis revealed design and execution limitations that cast doubts on the reliability of the results.9 In the recently published RADIANCE-HTN TRIO trial, patients with R-HTN treated with a standardized triple combination pill experienced a drop in their BP levels 2 months after RSD compared to a sham procedure.7 If the BP lowering effect and the safety of RSD are maintained in the long term, RSD might be an alternative to the addition of more antihypertensive medications in patients with R-HTN.
Renal sympathetic denervation in patients with uncontrolled hypertension
The new evidence available introduces a paradigm shift for a technique that was initially conceived for the management of R-HTN when all other therapeutic options fail, and is currently an option that should be taken into consideration in patients with persistent BP > 140/90 mmHg despite drug treatment.
The concept of uncontrolled HTN includes a high percentage of hypertensive patients (maybe even > 60%) with highly heterogenous clinical characteristics and cardiovascular risk. Given the invasive nature of the RSD procedure, and until more information becomes available on the reduction of cardiovascular events in more specific subgroups of patients, there are some high-risk situations in which BP control is essential to reduce the risk of cardiovascular events:
a) Patients with frequent hypertensive crises. Hypertensive crises with SBP levels > 180 mmHg and/or DBP levels > 110 mmHg can cause brain, cardiac or microvascular damage. Emergency visits for hypertensive crises exceed 4% of all visits to the emergency room.11 Even in the absence of hypertension-mediated organ damage (HMOD), episodes of hypertensive crisis can have long-term implications to the extent that these patients may have a 50% higher risk of suffering cardiovascular events compared to controlled hypertensive patients. Nonetheless, outside the crisis setting they show similar BP levels.12
b) Patients with low compliance to pharmacological treatment. Pharmacological treatment of HTN is generally a long-term option and in most cases, for life. Poor compliance is a common problem to the extent that almost one third of all hypertensive patients do not start a new prescription of antihypertensive drugs,13 and around 50% become non-compliant within the first year after starting treatment.13 In the SPYRAL HTN trials, the 24-hour ABPM levels showed decreased BP levels throughout the entire 24-hour period in patients treated with RSD compared to no changes in the control group in the absence of drugs or incomplete control in the presence of drugs.4,5 Furthermore, in the SPYRAL HTN OFF-MED trial, the treatment group experienced an average reduction of 9.2 mmHg in office SBP levels.5 A meta-analysis of 123 studies including 613 815 patients showed that a drop of office SBP levels of 10 mmHg was associated with a significantly lower risk of cardiovascular events.14 Poor compliance is a serious problem of public health since these patients in whom an adequate BP control is not achieved, even due to poor therapeutic compliance, have a high cardiovascular risk.15 However, we should stress that RSD alone cannot bring BP levels down enough to achieve BP control in most patients. In the RADIANCE-HTN SOLO trial, the 24-h ABPM only 25% of the patients treated with RSD reached values < 130/80 mmHg.6 In these non-compliant patients, the main strength of RSD is the “always on” effect regardless of pharmacokinetics and compliance to drugs.
c) Patients with hypertension-mediated organ damage. The presence of HMOD identifies a group of patients with high cardiovascular risk in whom conventional treatment has failed to prevent the progression of the disease.16 Achieving the BP levels recommended is especially important in these patients because, in the early stages of the disease, some types of HMOD can be reversed; in more advanced stages, HMOD is irreversible despite adequate BP control. But this is important since it slows its progression while reducing the cardiovascular risk of these high-risk patients.17 A meta-analysis including 698 patients treated with RSD revealed an independent effect of RSD on HMOD, which advocates for the use of RSD in this group of high-risk patients.18
d) Patients at high cardiovascular risk. The European guidelines on the management of HTN establish the factors that influence cardiovascular risk in hypertensive patients including clinical characteristics, analytical characteristics, presence of HMOD or established cardiovascular or kidney disease. All these factors establish a 10-year cardiovascular risk that is categorized into 4 groups: low, moderate, high or very high risk to the extent that, for example, in high-risk patients the estimated cardiovascular mortality is 5% and in very high-risk patients > 10%.8 The assessment of cardiovascular risk should play an important role in the decision-making process to the extent that the higher the risk, the greater the benefits expected with better BP control. Therefore patients at high or very high-risk would be eligible for RSD whenever BP control is not adequate.
Empowering the hypertensive patient in the setting of a shared decision-making process
Over the last few years, shared decision-making process has emerged as the go-to model in the management of different conditions. In the field of RSD, a recent survey revealed that 38% of hypertensive patients who still don’t take antihypertensive medication would prefer RSD to lifelong drug therapy even knowing that it would probably not replace medication in many cases. Just this already reduces BP significantly.19 With the evidence provided in recent trials, RSD could be a valid treatment option in patients with uncontrolled HTN and high to very-high cardiovascular risk in whom, in a shared decision-making process context, consensus with the patient can be reached. In any case, we should mention that the treatment of HTN always requires the adoption of healthy lifestyle habits, and the recommendation to patients should include drug treatment as the first option.
STUDY PRIOR TO RENAL SYMPATHETIC DENERVATION
Patients should be examined in a unit specialized in HTN and vascular risk 3 months prior to the procedure in a center with proven experience.20 Table 1 summarizes the studies to be conducted in patients eligible for RSD.
Uncontrolled HTN should be confirmed through 24-hour ABPM.21 After confirming the presence of uncontrolled HTN, the clinical situations that increase BP levels such as obesity or obstructive sleep apnea should be identified and corrected. Also, substances such as salt or certain drugs that may also lead to HTN should be suspended or minimized. Non-compliance to treatment, which is very common and not always identified by the patient, if not rigorously investigated, should be ruled out.22 It is essential to rule out secondary HTN (table 2) or, if diagnosed, treat it effectively. Still, it is not an absolute contraindication to RSD.23
RENAL SYMPATHETIC DENERVATION PROCEDURE WITH RADIOFREQUENCY DEVICES
Section 4 of the supplementary data shows more in-depth technical aspects of RSD. Figure 1 of the supplementary data summarizes the RSD procedure.
A better knowledge of the anatomy of renal nerves24 and the development of new ablation devices have optimized the treatment technique,5,6 which is based on 3 main objectives:
Figure 1. Detail of renal sympathetic innervation. Renal nerves are usually arranged in large bundles and only form a true plexus when they are close to entering the kidney. Some nerves bypass the main renal artery and join distally to the different arterial divisions of the main renal artery (late arrival nerves). In this case, a late arrival nerve is seen joining the proximal third of the anterior division of the main renal artery (blue asterisk). It can also be seen how the proximal main renal artery is occupied by fused ganglia of the solar plexus (GM), and by the lumbar sympathetic chain (LSC). Both provide innervation to the kidneys, but also to other abdominal and pelvic organs, which can be accidentally denervated if the proximal third of the main renal artery is treated. The image also shows that the maximum proximity of nerve fibers to the arterial wall mainly occurs at branch level, but also at main trunk level. This is the target area of treatment, always avoiding the application of radiofrequency at renal pelvis level. AG, adrenal gland; CT, celiac trunk; GM, ganglionic mass made up of the aorticorenal and celiac ganglia; LSC, lumbar sympathetic chain; RK, right kidney; SMA, superior mesenteric artery; blue asterisk, late arrival nerve. In red, arterial structures. In yellow, nervous tissue.
Management of the renal artery main trunk and branches
It is common for the renal nerves to reach the kidney after bypassing the main renal artery.24 In animal models, it has also been confirmed that the application of combined radiofrequency in the renal artery main trunk and branches reduced the content of norepinephrine in the renal tissue even more, and in the cortical axonal density, both associated with the response to RSD.25
In patients treated with RSD, the presence of untreated accessory arteries leads to a lower hypotensive response.26 Their identification and treatment is essential and, if they are amenable to treatment thanks to their diameter (minimum diameters of 3 mm), the treatment of accessory arteries is advised.
Last but not least, the perivascular space around the ostium and the proximal third of the main renal artery is often occupied by ganglia of solar plexus and by the lumbar sympathetic chain (figure 1). Both carry innervation to the kidneys, but also to other abdominal and pelvic organs, and they could be accidentally denervated if the treatment is applied to the ostium and proximal third of the main renal artery. Therefore, until more information becomes available, it seems reasonable to be cautious when treating the most ostial portion of renal arteries.24
Treatment of the 4 quadrants of the renal artery
The distribution of nerve fibers around the renal artery follows a variable pattern across different individuals.27 Preclinical studies in a porcine model have shown that the application of radiofrequency in one point produces effects on approximately 25% of the arterial circumference,27 and procedures that use multiple helically staggered ablations in the 4 quadrants are more effective reducing the norepinephrine content into the renal tissue.28
Application of the maximum possible number of ablation points
A post-hoc analysis of the SYMPLICITY HTN-3 trial confirmed that patients with a greater number of radiofrequency applications reduced their BP levels even more without any associated adverse events.9 We recommend applying the maximum number of ablation points possible, always respecting a distance of 5 mm among them with a 4-quadrant distribution.
Section 4 of the supplementary data shows how to perform a RSD procedure using a tetrapolar radiofrequency catheter. Table 3 shows the precautions and contraindications regarding RSD.
Care after renal sympathetic denervation procedure
Once the procedure is finished, it is important to ensure adequate hemostasis in the femoral puncture. Usually, in the absence of complications, patients can be discharged after 24 to 48 hours with the same antihypertensive treatment they had before the procedure or with treatment adjustments in cases that show an immediate response, but still with adjustment appointments within 5 to 7 days. Of note, the effects of the intervention can take weeks to materialize.29
CLINICAL MANAGEMENT AFTER RENAL SYMPATHETIC DENERVATION
The main objective of the follow-up should be to confirm the safety of the intervention and the absence of complications in the short-, mid-, and long-term follow-up, as well as to monitor the evolution of BP levels and the adjustment of drug treatment.
At the clinical follow-up, it is important to maintain a multidisciplinary team same as during the selection of candidates. Table 4 shows the clinical management after RSD.
Table 4. Clinical management after renal sympathetic denervation*
| Blood pressure control |
| Home self-measurement of blood pressure is recommended to assess blood pressure decrease |
| Patient education to detect symptoms of hypotension |
| Pharmacological de-escalation, when appropriate |
| 24-hour ambulatory blood pressure monitoring levels at 3-6 months to assess response to RSD |
| 24-hour ambulatory blood pressure monitoring levels to assess long-term durability of renal sympathetic denervation |
| Renal function: in patients at risk of contrast nephropathy, control should follow after 7-10 days (individualize based on the clinical criteria) |
| Routine renal imaging modalities (echocardiography, computed tomography scan, magnetic resonance imaging) are ill-advised |
* Control after renal sympathetic denervation should be performed in a hypertension- specific unit as part of a regulated renal sympathetic denervation program. |
REQUIREMENTS OF A RENAL SYMPATHETIC DENERVATION PROGRAM
The success of a RSD program is based on the existence of a multidisciplinary team that performs a comprehensive assessment of the patient from the selection of candidates through their assessment prior to the intervention, the RSD procedure, and subsequent follow-up. This process should be carried out at specific units specialized in the management of HTN in collaboration with interventional cardiology units. Figure 2 shows the selection process of eligible patients.
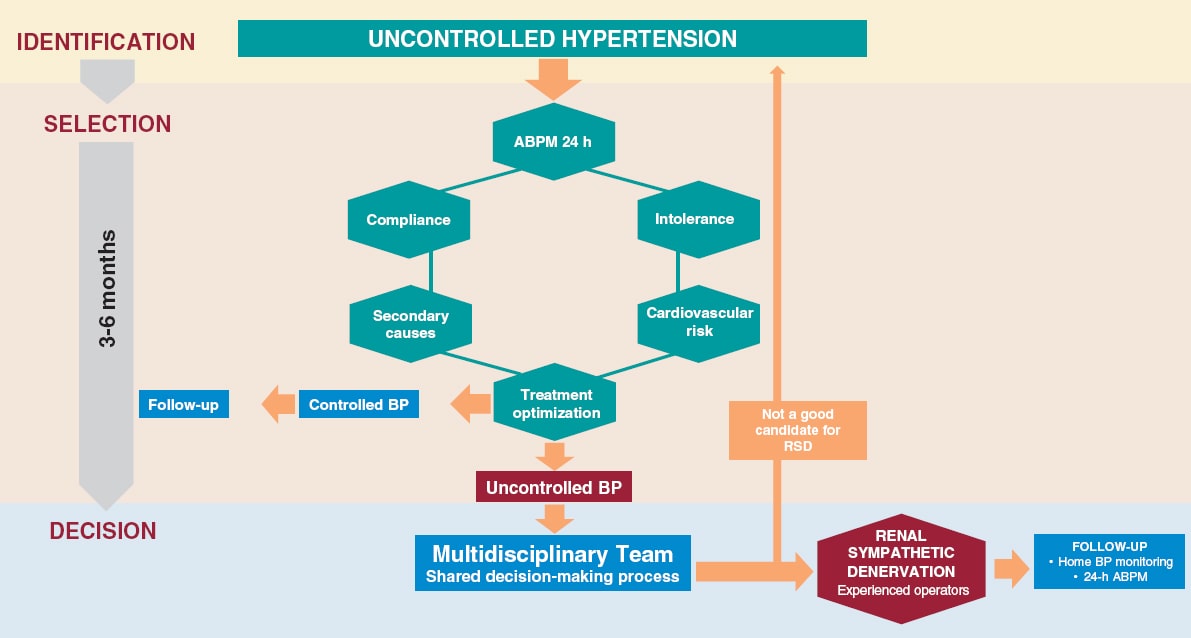
Figure 2. Identification process, patient selection and decision on RDN. Patients with uncontrolled HTN (BP > 140/90 mmHg despite treatment) should be evaluated in an HTN unit. The lack of control should be confirmed by ABPM, assess adherence/intolerance to drugs, rule out secondary causes and cardiovascular risk. If, after optimizing the treatment, the lack of control persists, in patients at high or very high risk, and in a shared-decision process with the patient, RDN may be indicated. Adherence is defined as the extent to which a person’s behavior —taking medication, following a diet, and/or executing lifestyle changes— corresponds with the agreed recommendations from a healthcare provider. Drug intolerance refers to an inability to tolerate the adverse effects of a medication, generally at therapeutic or subtherapeutic doses. Treatment optimization refers to lifestyle changes and pharmacological recommendations, including target doses, recommended by clinical practice guidelines.8 ABPM, ambulatory blood pressure monitoring; BP, blood pressure; RSD: renal sympathetic denervation.
We strongly discourage isolated procedures outside this controlled environment. RSD should not be performed in centers with volumes < 10 cases/year. Centers without a structured RSD program, but with eligible patients, should refer them to an experienced center rather than performing isolated procedures.
RSD procedures should be performed by operators experienced in the management of endovascular treatment. The SYMPLICITY HTN-3 trial post-hoc analysis showed the importance of an experienced interventional specialist given one of the factors influencing the results of the study was the operator’s lack of experience.9 Therefore, we recommend that procedures should be performed at centers with proven experience only and that, in centers that lack this experience, the possibility of monitoring should be available including assistance during the patient selection process and supervision of the procedure until enough experience is gained to ensure optimal results.
CONCLUSIONS
This expert consensus document has reviewed the information available regarding RSD in the management of patients with HTN. Also, it has established, for the first time, the indication for RSD in cases of uncontrolled HTN, especially in patients at high cardiovascular risk with HMOD or cardiovascular disease while taking the patient’s opinion into consideration as part of a shared decision-making process, and as long as it is evaluated by a multidisciplinary team and performed by experienced operators.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
Study concept and design: O. Rodríguez-Leor, and J.A. García-Donaire; manuscript writing: O. Rodríguez-Leor, F. Jaén-Águila, J. Segura, I. J. Nuñez-Gil, A. García-Touchard, E. Rubio, M. Troya, J. Diego-Mediavilla, and J.A. García-Donaire; critical review: O. Rodríguez-Leor, Á. Cequier, R. Moreno, N. Martell, P. Beltrán, and E. Molina.
CONFLICTS OF INTEREST
O. Rodríguez-Leor, and J. A. García-Donaire have received personal fees from Medtronic, outside the submitted work. A. García-Touchard reports having received grants from Medtronic, and personal fees from Medtronic, also outside the submitted work. Á. Cequier reports having received grants and personal fees from Abbott Vascular, grants, and personal fees from Biosensors, grants from Boston Scientific, grants, and personal fees from Medtronic, grants from Biomenco, Cordis, Orbus Neich, and from the Spain Society of Cardiology, personal fees from Ferrer International, Terumo, Astra Zeneca, and from Biotronik outside the submitted work. R. Moreno is associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. F. Jaén-Águila, J. Segura, I. J. Núñez-Gil, E. Rubio, M. Troya, J. Diego-Mediavilla, R. Moreno, N. Martell, P. Beltrán, and E. Molina declared no relationship whatsoever relevant to the contents of this paper worthy of disclosure.
REFERENCES
1. Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension:a multicentre safety and proof-of-concept cohort study. Lancet 2009;373:1275-1281.
2. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial):a randomised controlled trial. Lancet. 2010;376:1903-1909.
3. Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393-1401.
4. Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs:6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346-2355.
5. Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal):a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444-1451.
6. Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE HTN-SOLO):a multicentre, international, single-blind, randomised, sham controlled trial. Lancet. 2018;391:2335-2345.
7. Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO):a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476-2486.
8. Williams B, Mancia G, Spiering W, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology:ESH/ESC Task Force for the Management of Arterial Hypertension. Eur Heart J. 2018;39:3021-3104.
9. Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the Symplicity HTN-3 trial. Eur Heart J. 2015;36:219-227.
10. Mahfoud F, Böhm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes:3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40:3474–3482.
11. Janke AT, McNaughton CD, Brody AM, Welch RD, Levy PD. Trends in the incidence of hypertensive emergencies in US emergency departments from 2006 to 2013. J Am Heart Assoc. 2016;5:e004511.
12. Vleck M, Bur A, Woisetschläger C, Herkner H, Laggner AN, Hirschl MM. Association between hypertensive urgencies and subsequent cardiovascular events in patients with hypertension. J Hypertens. 2008;26:657-662.
13. Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence:analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25:284-290.
14. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death:a systematic review and meta-analysis. Lancet. 2016;387:957-967.
15. Gupta P, Patel P, Strauch B, et al. Risk factors for nonadherence to antihypertensive treatment. Hypertension. 2017;69:1113-1120.
16. Cordero A, Morillas P, Bertomeu-Gonzalez V, et al. Prevalence of Peripheral Arterial Disease in Patients with Acute Coronary Syndrome Investigators. Clustering of target organ damage increases mortality after acute coronary syndromes in patients with arterial hypertension. J Hum Hypertens. 2011;25:600-607.
17. Lonnebakken MT, Izzo R, Mancusi C, et al. Left ventricular hypertrophy regression during antihypertensive treatment in an outpatient clinic (the Campania Salute Network). J Am Heart Assoc. 2017;6:e004152.
18. Kordalis A, Tsiachris D, Pietri P, et al. Regression of organ damage following renal denervation in resistant hypertension:a meta-analysis. J Hypertens. 2018;36:1614-1621.
19. Schmeider RE, Hogerl K, Jung S, Bramlage P, Veelken R, Ott C. Patient preference for therapies in hypertension:a cross-sectional survey of German patients. Clin Res Cardiol. 2019;108:1331-1342.
20. Schmieder RE, Redon J, Grassi G, et al. ESH Position Paper:Renal denervation - an interventional therapy of resistant hypertension. J Hypertens. 2012;30:837-841.
21. Banegas JR, Messerli FH, Waeber B, et al. Discrepancies between office and ambulatory blood pressure:clinical implications. Am J Med. 2009;122:1136-1141.
22. Burnier M, Wuerzner G, Struijker-Boudier H, Urquhart J. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension. 2013;62:218-225.
23. Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension:when, who, and how to screen?Eur Heart J. 2014;35:1245-1254.
24. García-Touchard A, Maranillo E, Mompeo B, Sañudo JR. Microdissection of the Human Renal Nervous System:Implications for Performing Renal Denervation Procedures. Hypertension. 2020;76:1240-1246.
25. Mahfoud F, Tunev S, Ewen S, et al. Impact of lesion placement on efficacy and safety of catheter-based radiofrequency renal denervation. J Am Coll Cardiol. 2015;66:1766-1775.
26. Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation?JACC Cardiovasc Interv. 2013;6:1085-1091.
27. Tzafriri AR, Mahfoud F, Keating JH, et al. Procedural and anatomical determinants of multi-electrode renal denervation efficacy –Insights from preclinical models. Hypertension. 2019;74:546-554.
28. Tzafriri AR, Mahfoud F, Keating JH, et al Innervations patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64:1079-1087.
29. Fink G, Phelps JT. Can we predict the blood pressure response to renal denervation?Auton Neurosci. 2017;204:112-118.
* Corresponding author: Hospital Universitari Germans Trias i Pujol, Carretera de Canyet s/n, 08916 Badalona, Barcelona, Spain.
E-mail address: oriolrodriguez@gmail.com (O. Rodríguez-Leor).
ABSTRACT
Coronary vasoreactivity testing is a key diagnostic procedure in patients with suspected coronary spasm and research procedures intended to assess the coronary endothelial function. We should mention that coronary spasm has been observed in > 40% of the patients with angina and non-obstructive coronary stenosis. Also, that its dedicated treatment has proven to reduce ischemic symptoms and improve these patients’ quality of life. This technical report elaborated by the Working Group on Intracoronary Diagnostic Techniques of the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) summarizes the indications, preparation, performance, and interpretation of the vasoreactivity testing performed by intracoronary infusion of acetylcholine.
Keywords: Spasm provocation test. Coronary endothelial function.
RESUMEN
Las pruebas de vasorreactividad coronaria con infusión de acetilcolina son una prueba diagnóstica fundamental para pacientes con sospecha de enfermedad cardiaca secundaria a vasoespasmo y en procedimientos de investigación en los que se valora la función endotelial coronaria. Se calcula que más del 40% de los pacientes con angina y ausencia de lesiones coronarias presentan vasoespasmo como causa fundamental de los síntomas, y su tratamiento específico ha demostrado mejorar la calidad de vida en estos pacientes. El Grupo de Trabajo de Técnicas de Diagnóstico Intracoronario de la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (ACI-SEC) ha elaborado el presente documento técnico que expone de manera práctica las indicaciones, la preparación, la realización y la interpretación de dichas pruebas.
Palabras clave: Prueba de provocación de vasoespasmo. Función endotelial coronaria.
Abbreviations: DS: diameter stenosis. INOCA: ischemia with no obstructive coronary arteries. MINOCA: myocardial infarction with non-obstructive coronary arteries.
INTRODUCTION
Coronary vasoreactivity testing performed by intracoronary infusion of acetylcholine is basically used with 2 goals in mind: for endothelial function assessment and as a vasospasm provocation test in clinically suspicious cases. Although these tests have been known and used for decades, its use is not yet fully consolidated in our setting. This is mainly due to a scarce suspicion of myocardial ischemia due to micro or vasomotor disorders that has eventually lowered the demand for these tests. In addition, the lack of test standardization and training, the off-label use of acetylcholine, and the doubts surrounding these tests safety profile have not encouraged their widespread use in the routine clinical practice.
Over the last few years, this scenario has changed dramatically thanks to the growing evidence on the importance of diagnosing the causes of myocardial ischemia not directly related to fixed stenoses. Currently, invasive coronary spasm provocation tests are formally recommended by the European Society of Cardiology in its clinical practice guidelines on the management of chronic coronary syndromes, non-ST-segment elevation acute coronary syndromes, and ST-segment elevation acute coronary syndromes.1-3 The most common indications are to treat patients with angina or ischemia but without non-obstructive coronary lesions (ANOCA, INOCA—in this document, both coined under the term INOCA—), myocardial infarction with non-obstructive coronary arteries (MINOCA), persistent angina after coronary revascularization, obstructive coronary artery disease with clinical suspicion of associated angina of microvascular origin, and finally, patients with recovered sudden death of undetermined causes.1-3 Table 1 summarizes all clinical indications and level of recommendation to perform vasospasm provocation testing. Although this paper focuses on coronary vasoreactivity testing, we should remember that its use is often recommended simultaneously with other coronary functional testing performed using pressure guidewires like coronary flow reserve and microcirculation resistance measurements.1-5 The specific diagnosis of functional damage to coronary arteries and its targeted therapies have both improved the quality of life of patients with INOCA.6 The treatment recommended for coronary vasospasm is calcium channel blockers, nitrates, and nicorandil.6,7
Table 1. Clinical indications for the coronary vasospasm provocation test performed by intracoronary infusion of acetylcholine
| Class | Indication | Clinical specifications |
|---|---|---|
| Class I (highly recommended) | Clinical suspicion of vasospastic angina without objective documentation of ischemia or obstructive coronary artery disease in patient with chronic symptoms | – Vasospastic angina can occur predominantly at rest (35%), during exertion (30%), as a mixed pattern (30%) or dyspnea (5%)4 – The epicardial and microvascular function assessment in maximum hyperemia with a pressure guidewire is advised |
| Acute coronary syndrome without presence of culprit lesions on the coronary angiography | – Carefully review the angiography to discard embolisms and radiolucent images consistent with thrombus or coronary dissections – The use of intravascular imaging (intracoronary ultrasound or optical coherence tomography) for this type of lesions is advised – Exclude other causes for high troponin levels (like myocarditis) through segmental assessments (ventriculography or echocardiography) and magnetic resonance imaging | |
| Recovered inexplicable sudden death | – After excluding structural and/or arrhythmic heart disease | |
| Study of syncope preceded by thoracic pain | – After excluding structural and/or arrhythmic heart disease | |
| Recurrent angina despite revascularization | – First assess the pressure guidewire to exclude epicardial functional disease and microcirculation disorders in maximum hyperemia | |
| Class IIa (recommended) | Clinically documented vasospastic angina in a spontaneous event or on the non-invasive provocation test that is unresponsive to medical therapy | – Patients unresponsive to therapy with calcium channel blockers and nitrates or nicorandil – Epicardial and microvascular function assessment in maximum hyperemia with a pressure guidewire is advised |
| Class IIb (debatable) | Clinically documented vasospastic angina or on the non-invasive provocation test that responds to medical therapy to know the type and degree of vasospasm | – The specification of the macro/microvascular spasm and whether it causes the occlusion of the artery can be relevant for the patient’s prognosis – Epicardial and microvascular function assessment in maximum hyperemia with a pressure guidewire is advised |
| Class III (ill-advised) | Asymptomatic patients | Asymptomatic patients |
| Patients with ejection fractions < 35% | Patients with ejection fractions < 35% | |
| Significant epicardial coronary artery disease (left main coronary artery and/or 3 vessels) | Significant epicardial coronary artery disease (left main coronary artery and/or 3 vessels) | |
Adapted with permission from the consensus document elaborated by the COVADIS Working Group (Coronary Vasomotion Disorders International Study).5 | ||
Based on the current clinical practice guidelines, the objective of the Working Group on Intracoronary Diagnostic Techniques of the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) is to facilitate and standardize the use of coronary vasoreactivity testing. Thus, this paper has been drafted to expose all technical steps in a practical way to encourage the performance and interpretation of these tests in our setting.
NORMAL ENDOTHELIAL FUNCTION OF CORONARY ARTERIES
The modulatory function of vascular endothelium in the blood flow towards the myocardium is intrinsically associated with its metabolic characteristics. Compared to the skeletal muscle, the heart has pretty high oxygen needs (some 20 times higher). The way this oxygen supply is achieved is through very high tissue extraction at baseline: at rest the myocardium extracts approximately 70% to 80% of the oxygen transported by hemoglobin compared to 30% of the skeletal muscle. This explains why, unlike other organs, the mechanism through which the heart regulates oxygen supply to the myocardium changing metabolic needs is fast regulation and constant blood flow into the coronary system.8
Microcirculation (arteries and arterioles < 400 µm) is basically responsible for regulating coronary blood flow. Although regulation is complex and includes metabolites, hormones, neurotransmitters, and other factors, the main protagonist is the vascular endothelium that produces nitric oxide—a powerful vasodilator—in response to different stimuli. Also, other vasodilator factors—like the hyperpolarizing endothelial factor—and vasoconstrictor factors like endothelin. Endothelium-dependent vasodilation can be stimulated through different ways, but the most commonly used one is the infusion of acetylcholine.
In normal conditions, an artery with a healthy endothelium responds to acetylcholine by releasing nitric oxide that translates into vasodilation. In the presence of artery denudation from the endothelium or if the action of the nitric-oxide synthase enzyme is blocked, the artery responds to acetylcholine with vasoconstriction due to the stimulation of smooth muscle muscarinic receptors not counteracted by the nitric oxide of endothelial origin. Therefore, the infusion of acetylcholine can be used to assess endothelial function: if normal, vasodilation becomes evident. If not, vasoconstriction kicks in. The macrovascular compartment endothelial function (epicardial) can be assessed on an angiography. However, to assess the endothelium-dependent response in microcirculation, blood flow should be measured using a Doppler guidewire or thermodilution. From the macrovascular point of view, visually evident epicardial vessel vasoconstriction in response to acetylcholine is considered endothelial dysfunction. Figure 1 shows examples of vasodilation (physiological) and vasoconstriction responses (suggestive of endothelial dysfunction) to the administration of acetylcholine. From the microvascular point of view, flow reductions or increases < 50% in response to the administration of acetylcholine are considered anomalous.9 Figure 2 shows examples of microvascular function assessment using the Doppler technique or intracoronary thermodilution.
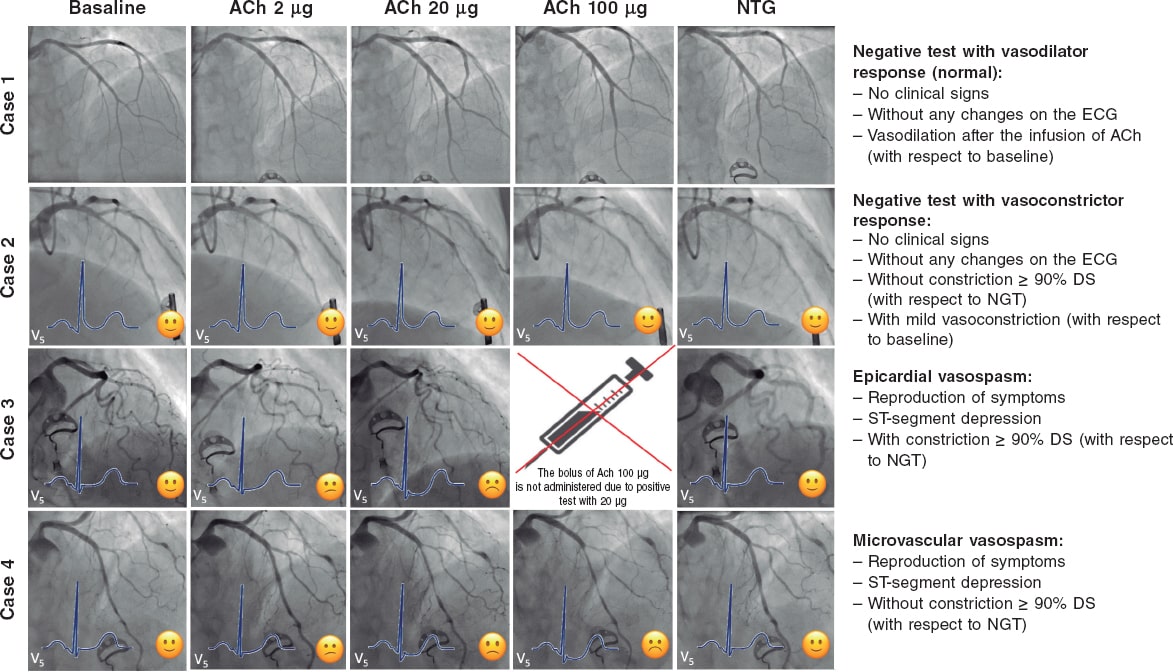
Figure 1. Possible results of the vasospasm provocation test with acetylcholine. Case 1: physiological response (vasodilator response to acetylcholine). Case 2: endothelial dysfunction with vasoconstriction with respect to baseline that does not meet the criteria for micro or macrovascular spasm. Case 3: macrovascular spasm with significant vasoconstriction of the left coronary tree. Case 4: microvascular spasm due to moderate vasoconstriction of the left tree meeting the clinical and ECG criteria for ischemia. ACh, acetylcholine; DS, diameter stenosis; NTG, nitroglycerin.
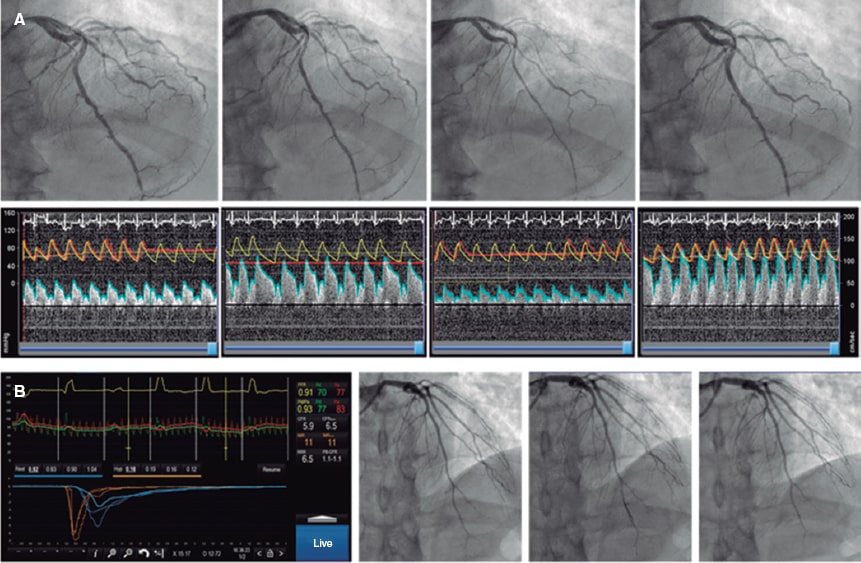
Figure 2. Combined assessment of macro and microvascular function. A: pressure-Doppler guidewire study (Combowire, Philips, The Netherlands). After the angiography and measurement of baseline flow velocity, growing doses of acetylcholine are injected. After the second dose, moderate vasoconstriction of the left anterior descending coronary artery occurs followed by an occlusive spasm at circumflex artery level plus a slower flow velocity in the left anterior descending coronary artery indicative of microvascular vasoconstriction. The spasm is solved with intracoronary nitroglycerin followed by the adenosine non-endothelium-dependent assessment of microvascular function. The patient shows macro and microvascular endothelial dysfunction and a normal non-endothelium-dependent microvascular function. B: macrovascular endothelial function assessment appears normal; adenosine non-endothelium-dependent assessment with thermodilution guidewire (Pressurewire X, Abbott, United States). Coronary flow reserve is 5.9, and the index of microcirculatory resistance is 11, which is suggestive of a normal microvascular function. In conclusion, physiological examination without any relevant findings.
CORONARY VASOSPASM PROVOCATION TESTING
Different stimuli can be used to provoque epicardial or microvascular coronary spasm. Non-pharmacological stimuli like hyperventilation or coming into contact with cold are associated with an excessive number of false negatives for clinical use. Non-invasive coronary vasospasm assessment (based on changes on the ECG or the echocardiography through the IV administration of ergonovine) is associated with a risk of causing nitrate-resistant flow-limiting coronary spasm.4 For this reason, to this date, invasive studies based on the intracoronary administration of drugs are considered the single most sensitive and safe method. Actually, to this date, it is the method recommended by European guidelines and consensus docments.2,7 The direct administration of drugs allows us to use lower doses and establish a time correlation between the development of coronary spasm followed by symptom onset and changes on the ECG. Also, it facilitates immediate treatment through the direct administration of nitrates.4,10 The use of acetylcholine vs ergonovine is advised too since the former acts on a specific pathway (by stimulating cholinergic receptors only), and its safety profile is good because its half-life is shorter. Also, because it responds faster to nitrates in case of vasoconstriction.11 Also, acetylcholine allows us to assess the vascular endothelial response specifically, which is an additional advantage. The studies that compared the results of vasospasm provocation testing with acetylcholine vs ergonovine found similar sensitivity and high matching (94%) between the two. Therefore, in the presence of a negative acetylcholine test no additional studies with different drugs are advised.12
ACETYLCHOLINE
Acetylcholine is a neurotransmitter largely found in the nervous system (central, autonomous, and peripheral). It is used in the neuromuscular junction, in all synapses of the parasympathetic autonomous system, and in the first synapsis of the sympathetic nervous system. The muscarinic receptor of acetylcholine has 5 different subtypes; among them, subtype M2 is largely found in the myocardium where it reduces the heart rate and the cardiac conduction system; subtype M3 is found in the coronary arteries both in the endothelium and the smooth muscle. In the coronary arteries, the M3 receptor stimulates the contraction of the vascular smooth muscle (vasoconstriction). Also, it stimulates the endothelial production of nitric oxide that spreads into the smooth muscle reducing the concentration of calcium, and causing relaxation (vasodilation).13,14 Acetylcholine is rapidly hydrolyzed in both the neuromuscular junction and blood by the action of cholinesterases. When infused intracoronary in the doses described here, no systemic effects occur, and its cardiac effects only last a few minutes.
ACETYLCHOLINE-INDUCED ENDOTHELIAL DYSFUNCTION AND CORONARY VASOSPASM
Endothelial dysfunction is associated with the number of cardiovascular risk factors and is a well-known precursor of atherosclerosis.15 Also, the presence of endothelial dysfunction has been associated with the appearance of ischemia in the ischemia exercise test, heavier calcification and presence of necrotic and lipidic content in the vascular wall, and more cardiovascular adverse events in the long run.16-18 The prevalence of a vasoconstrictor response to the intracoronary infusion of acetylcholine, therefore, similar to an epicardial endothelial dysfunction, is variable depending on the characteristics of the patients being more common among males.19 In the studies conducted in patients with INOCA, the prevalence of endothelial dysfunction is somewhere between 45% and 75%.19,20
Although, to this point, no cut-off value has been universally accepted, it has been confirmed that moderate degrees of vasoconstriction (20% to 50%) with respect to the artery baseline diameter after the intracoronary infusion of acetylcholine have an important prognostic impact.18,21,22 Quantitative coronary angiography studies consider the variability of the technique when measuring changes in the mean luminal diameter of a segment with respect to the different doses of acetylcholine (usually the dose with the highest vasoconstriction with respect to the baseline one). Small imaging variations due to respiratory movements in every cine coronary arteriography, different limits of the study segment in the different measures taken, the analysis of diameters at different times of the cardiac cycle between the baseline image and maximum vasoconstriction, and the operator’s variability are the reasons why vasoconstriction can only be confirmed after variability is excluded from this measuring process. Several studies have established this variability (2 times the standard deviation of the percent difference) somewhere between 3% and 6%. Therfore, endothelial dysfunction is defined as a vasoconstrictor response that is greater than this variability.23,24
The pathophysiological factors of vasospastic angina, both in their macro and microvascular manifestations are less known and, also, probably multifactorial. Vasospastic angina has been associated with the presence of coronary plaques, vascular smooth muscle cell hyperreactivity, a high baseline vagal tone, hyperreactivity to sympathetic stimulation, and finally, to a significant degree of endothelial dysfunction.10 Vasospastic angina, both macro and microvascular, is more common among women.4 The traditional criteria to define vasospastic angina of macrovascular origin have been described by the Coronary Vasomotion Disorders International Study Group (COVADIS).5 In their document they describe the diagnostic criteria of this disease that go beyond the traditional definition of variant angina described by Prinzmetal et al.25. We should mention that, unlike the definition of endothelial function where baseline angiography is used as the reference, to define macrovascular spasm the COVADIS group recommends assessing the coronary spasp in the segment with the greatest constriction of all after the administration of acetylcholine and then compare it with the diameter of the same segment after the infusion of nitroglycerin.4 Also, this group recommends the use of drug provocation testing performed by intracoronary infusion of acetylcholine given its high sensitivity and specificity values (90% and 99%, respectively).26 Based on former studies and the traditional definition, the prevalence of epicardial coronary artery vasospasm, whether associated with microvascular spasm or not, occurs in 30% to 40% of the patients with INOCA.6,27
Fewer consensus documents have been published on the definition and diagnosis of microvascular spasm.28,29 Over the last few years, the appearance of thoracic pain and changes on the ECG suggestive of ischemia in response to acetylcholine and in the absence of macrovascular spasm have been accepted for the diagnosis of microvascular spasm (located in the arterioles). By this definition, 25% of the patients with INOCA meet the microvascular spasm criteria.27
TEST PERFORMED BY INTRACORONARY INFUSION OF ACETYLCHOLINE
Preparing the patient
The best way to prepare patients eligible for the coronary vasoreactivity test with acetylcholine is still under discussion. Historically, these procedures used to be performed in a dedicated procedure while avoiding and withdrawing all kinds of vasodilator drugs (like calcium channel blockers and nitrates) for, at least, 18 hours before the infusion of acetylcholine.27,30,31 However, after the publication of the randomized clinical trial CorMicA and the consensus document of the European Association of Percutaneous Coronary Interventions (EAPCI) on the study of patients with INOCA, conventional wisdom has changed.6,7 Currently, the use of intracoronary functional testing is recommended including the vasoreactivity test to acetylcholine within the same diagnostic procedure where the coronary angiography is performed.
This brings greater comfort to the patient, uses cath lab resources more efficiently, and alleviates the pressure of the hospital agenda. In any case, this procedure should be fully adapted to the needs and possibilities of every cath lab; in polymedicated patients with vasodilators or in inexperienced centers using this test the scheduled procedure should be used.
If radial access is used in patients eligible for a coronary vasoreactivity test the administration of calcium channel blockers to prevent radial spasm is ill-advised. In these cases, the administration of low doses of nitroglycerin through the introducer sheath (100 µg to 200 µg) can be considered. However, its effect will probably mostly be gone by the time acetylcholine is infused. Also, the coronary vasoreactivity test can be performed after studying microvascular function with a pressure guidewire (with the corresponding administration of intracoronary nitroglycerin before advancing the guidewire).6,7 In this case, a 2- to 3-min washout period should be observed before the infusion of acetylcholine.6,7
Finally, a specific informed consent should be obtained before running any vasoreactivity tests. Also, 12-lead ECG monitoring is required to assess the results. The use of radiotransparent wiring and electrodes is advised here to avoid interfering with the cine-fluoroscopy images obtained for each of the doses infused during coronary angiography. Figure 3 shows a schematic sample of how to prepare a patient before running a coronary vasoreactivity test with intracoronary acetylcholine.
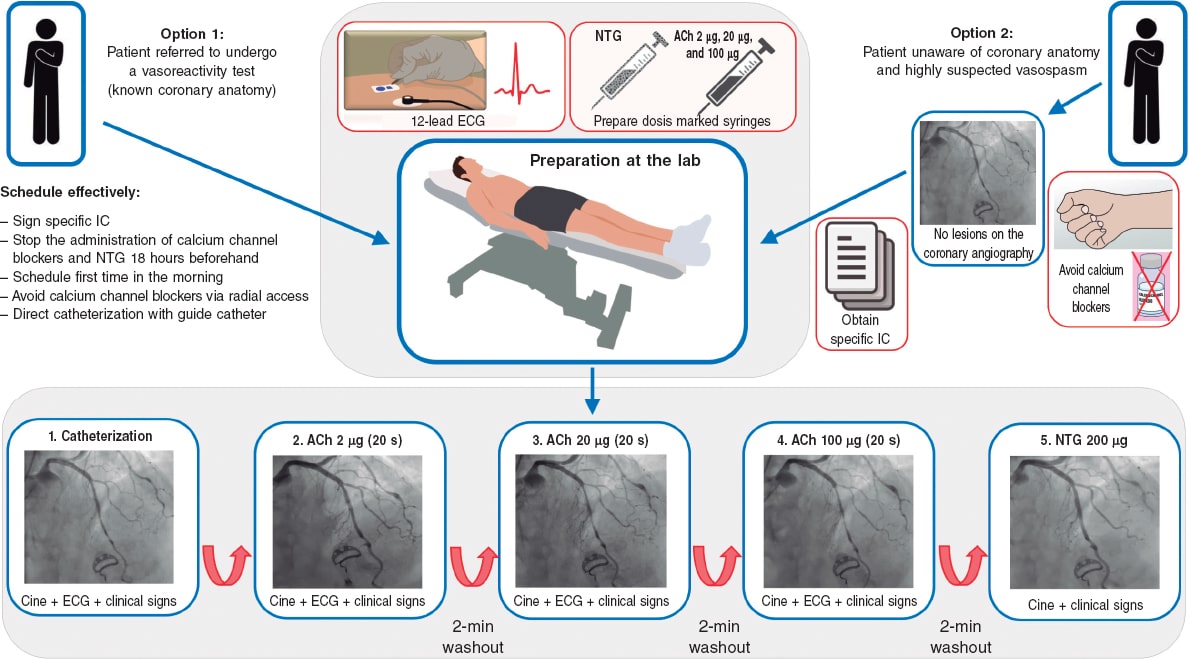
Figure 3. Preparation of the patient before running any vasoreactivity tests. ACh, acetylcholine; Ca, calcium; Cine, cine coronary arteriography; IC, informed consent; NTG, nitroglycerin.
Regarding the use of beta-blockers, certain groups also recommend their cessation before running the test to avoid any possible vasoconstrictor effects. Until more scientific data become available, the opinion of this group is that beta-blockers do not affect the results of the test significantly and in no way give false negative results.
Preparing the acetylcholine
The acetylcholine available in Spain is a preparation for the intraocular injection of 20 mg of acetylcholine chloride (powder) for its dilution in a 2 mL vial of physiological saline solution. After the solution has been prepared, the drug is still unstable, which is why the best thing to do is to prepare it right before the test; if several consecutive tests are going to be performed, the same preparation can be used. Figure 4 summarizes the way to prepare the acetylcholine solutions suggested for the test. An important safety tip is to identify correctly every solution of acetylcholine we will be using; saline solution systems and color syringes for every dose can be useful.
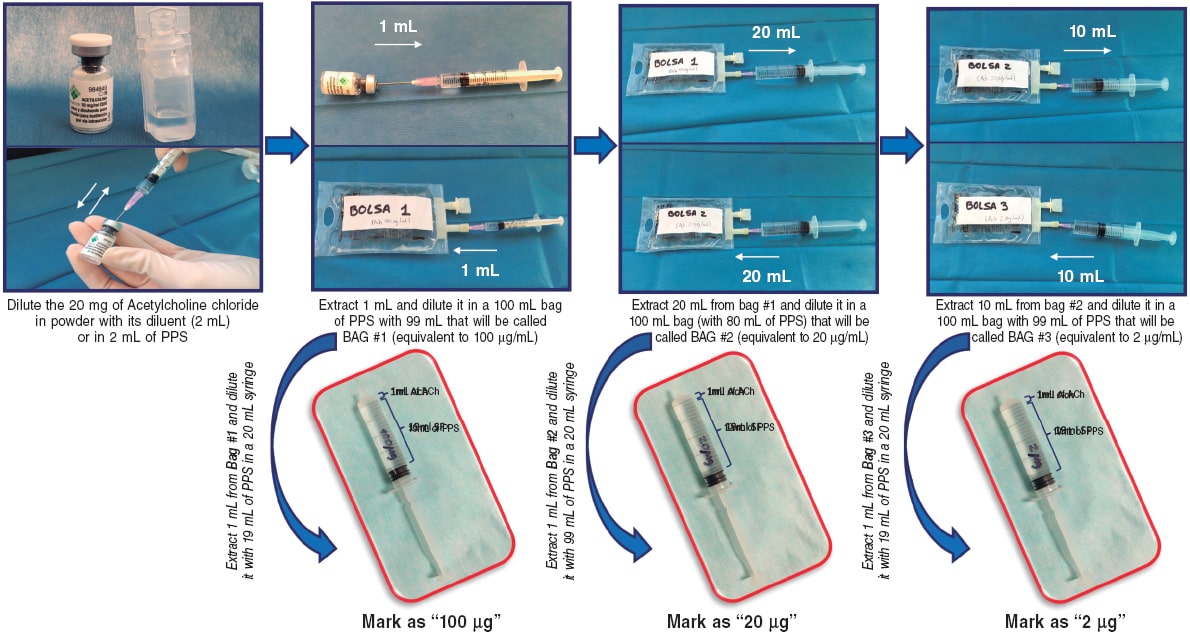
Figure 4. Preparation of growing doses of acetylcholine. ACh, acetylcholine; DS, diameter stenosis; PSS, physiological saline solution.
Intracoronary infusion protocol
Over the last 30 years, different protocols on the administration and doses of intracoronary acetylcholine have been used. Table 2 shows the different protocols used in landmark studies.6,10,19,29,30,32-34 There are differences regarding the routes of administration (manual infusion through guide catheter or controlled selective infusion into an artery through an infusion pump and microcatheter), the number of doses infused (from 2 to 4), the amount of acetylcholine used (from 0.3 µg to 200 µg), and the infusion time (from 20 seconds to 3 minutes). Below we will be seeing the most widely accepted protocols based on the objective pursued (endothelial function assessment or vasospasm provocation) followed by a proposal according to the last consensus documents published to this date.
Table 2. Comparison of the different protocols of coronary vasoreactivity to acetylcholine
| Group | Infusion method | Doses used | Infusion time per dose | Comments |
|---|---|---|---|---|
| Harvard Working Group30 | Infusion through microcatheter and infusion pump | 4 dilutions of 10–7, 10–6, 10–5, and 10–4 per liter (infusion at a rate of 0.8 mL/min) into the LCA | 2 minutes | – Designed for endothelial function assessment – A final concentration of 10–9, 10–8, 10–7, and 10–6 is estimated (equivalent to a selective total dose per artery of 0.03 µg, 0.3 µg, 3 µg, and 30 µg) – It is performed on the LCA |
| Mayo Clinic32 | Infusion through microcatheter and infusion pump | 3 dilutions of 10–6, 10–5, and 10–4 per liter (infusion at a rate of 1 mL/min) followed by a bolus of 100 µg (through the same catheter) | 3 minutes (final bolus for 20 to 30 seconds) | – Mixed protocol for endothelial function assessment (equivalent to a selective total dose per artery of 0.5 µg, 5 µg, and 50 µg) and vasospasm assessment with a bolus of 100 µg – It includes the functional assessment of microcirculation with Doppler guidewire during the infusion of acetylcholine – It is performed on the LCA |
| Korea Working Group33 | Manual infusion through guide catheter | 3 doses of 20 µg, 50 µg, and 100 µg into the LCA | 1 minute | – It is performed on the LCA |
| Japanese Circulation Society10 | Manual infusion through guide catheter | 3 doses of 20 µg, 50 µg, and 100 µg into the LCA In the absence of vasospasm 2 doses of 20 µg and 50 µg into the RCA are advised | 20 seconds | – Vasospasm provocation test on the LCA and RCA – The implantation of an electrode catheter to perform it is advised |
| Standford Working Group19 | Manual infusion through guide catheter | 4 doses of 20 µg, 50 µg, 100 µg, and 200 µg into the LCA | 1 minute | – It is performed on the LCA |
| Stuttgard Working Group34 | Manual infusion through guide catheter | 4 doses of 2 µg, 20 µg, 100 µg, and 200 µg into the LCA In the absence of vasospasm into the LCA an 80 µg dose into the RCA is advised | 20 seconds | – It studies both the LCA and the RCA |
| The CorMicA trial and the COVADIS Working Group6,29 | Mixed pump and manual infusion | 3 growing doses of 0.18 µg/mL, 1.82 µg/mL, and 18.2 µg/mL administered using an infusion pump through the guide catheter The procedure is completed with a manual bolus of 100 µg (50 µg into the RCA) | 2 minutes for every growing dose, and 20 seconds for the final bolus | – It is performed on the LCA after microcirculation assessment with adenosine through a pressure guidewire – It assesses the endothelial function and the vasospasm provocation test in the same procedure |
| Protocol of the ACI-SEC (present document) | Manual infusion through guide catheter | 3 doses of 2 µg, 20 µg, and 100 µg into the LCA In case of suspected vasospasm into the RCA the test should be started in this artery with doses of 2 µg, 20 µg, and 50 µg | 20 seconds | – For endothelial function assessment purposes, the doses should be infused more slowly for 2 to 3 minutes – It is performed on the LCA |
ACI-SEC, Interventional Cardiology Association of the Spanish Society of Cardiology; RCA, right coronary artery; LCA, left coronary artery. | ||||
Endothelial function assessment
Growing doses of acetylcholine are used for endothelial function assessment. If this procedure is performed by selective drug infusion into 1 of the main coronary vessels with a microcatheter (usually the left anterior descending coronary artery), the concentrations used are 10 moL/L to 6 moL/L, 10 moL/L to 5 moL/L, and 10 moL/L to 4 moL/L. Considering the left anterior descending coronary artery flow (some 80 mL/min), it is estimated that the drug reaches concentrations that are 100 lower in coronary microcirculation. Using the microcatheter these dilutions are injected the into the proximal left anterior descending coronary artery or into the artery to be interrogated at a rate of 1 mL/min for 3 minutes or 2 mL/min for 2 minutes through an infusion pump.24,35 Infusion starts with the least concentrated dilution and, if no complications or overt vasospasm are reported the next infusion should start 2 to 3 minutes later. In practice, this method injects 0.5 µg, 5 µg, and 50 µg of acetylcholine in each of the doses. As already mentioned, in the presence of a non-dysfunctional vascular endothelium, the physiological response is the vasodilation of major epicardial vessels.
The procedure described, although widely used in clinical trials, is somehow complicated and expensive, which is why easier and more practical alternatives have been developed for macrovascular endothelial function assessment. The most important one that has already become the standard may be the one used in the ENCORE trials (Evaluation of nifedipine and cerivastatin on recovery of coronary endothelial function)36,37 consisting of the infusion of growing doses of 2 µg, 20 µg, and 100 µg directly into the left main coronary artery for 3 minutes each followed by the performance of an angiography after every dose. Also, it consists of the assessment of the arterial diameter compared to the one measured on the baseline angiography. If macrovascular endothelial function needs to be assessed, the recommendation is to follow this infusion pattern. As we will be seeing, this protocol has already been widely adopted in recent publications and, with minor changes, has become the go-to protocol for the diagnosis of coronary vasospasm although with a faster infusion of the doses.
Microvascular endothelial function can also be assessed using dedicated guidewires for the simultaneous measurement of coronary flow. In general, this procedure is performed using a Doppler guidewire (Combowire, Philips, The Netherlands),9 although the assessment can also be performed through thermodilution with thermistor-based temperature measuring guidewires (Pressurewire, Abbott, United States).38,39 Figure 2 shows 2 examples of this procedure.
Coronary spasm provocation testing
Although there are different protocols on doses and infusion times, the protocol recommended here for vasospasm provocation has been widely accepted by the most experienced groups. In addition, there are data available on its safety profile in many patients and is the protocol backed by the EAPCI in its recent consensus document.7
Three doses of 2 µg, 20 µg, and 100 µg are used in the left coronary artery, and 3 doses of 2 µg, 20 µg, and 50 µg in the right coronary artery. If the test is negative or inconclusive and the previous doses are well-tolerated, a 200 µg or a 80 µg dose can be used in the left or right coronary artery, respectively, if suspicion runs high.
Regarding the infusion time, a slow 20 second-bolus can be administered, although this is based on clinical tolerability. The highest doses, especially in the right coronary artery, often require infusions at a slower rate to avoid sinus arrest-induced bradycardia or atrioventricular block. It is important to carefully and slowly wash the guide catheter with a saline solution to avoid the sudden injection of the remaining drug into the catheter by the time the cine-fluoroscopy imaging is acquired. After every dose both the symptoms and the repolarization and angiographic changes should be assessed while paying special attention to the appearance of epicardial spasms or significant reductions of coronary flow velocity. At the end of the test, intracoronary nitroglycerin is infused (200 µg to 300 µg) and spasm is solved within a few seconds.
Safety and complications
Before indicating the test, the presence of factors that may be correlated with a risk of complications associated with the intracoronary infusion of acetylcholine should be discarded. The test should be carefully performed in patients with a past medical history of asthma or bronchospasm and serious disorders of automaticity and cardiac conduction.
Although safe in experienced hands, coronary vasoreactivity tests to acetylcholine are not stranger to potentially serious complications. These tests should always be performed paying extra care by trained personnel and ready to face the possible complications that may arise. In a metanalysis of different studies with over 6000 procedures, the rates of major (eg, ventricular arrhythmias, need for cardiopulmonary resuscitation or infarction), and minor complications (symptomatic bradycardia, transient atrioventricular block, appearance of ventricular arrhythmias or air embolism) were 1% and 6%, respectively.40 We should mention that no death was reported in this metanalysis.40 Table 3 shows the most common complications and their corresponding treatments.
Table 3. Complications associated with the intracoronary infusion of acetylcholine
| Complication | Percentage | Comment | Treatment |
|---|---|---|---|
| Bradycardia and/or transient atrioventricular block | 3.23% | More common in high doses and when infused fast, especially into the RCA | Stop the infusion for a few seconds until going back to rhythm. Study the possibility of going on with the test at a slower infusion rate |
| Appearance of atrial fibrillation | 2.38%* | It is often self-limiting, but also fast, and its clinical tolerance is poor. It is a reason to stop the test whose outcome will be undetermined | If hemodynamic tolerance is good, use antiarrhythmic drugs; if poor, study the possibility of electrical cardioversion |
| Ventricular fibrillation, ventricular tachycardia or need for resuscitation | 1.00% | Due to acute ischemia following flow-limiting vasospasm | Nitroglycerin and defibrillation |
| Shock and/or myocardial infarction | 0.07% | Due to flow-limiting spasm at multivessel or left main coronary artery level | Nitroglycerin plus inotropic support +/– ventricular support |
| Transient hypotension | 0.05% | It is often unsignificant | Stop the infusion for a few seconds until going back to rhythm. Study the possibility of going on with the test at a slower infusion rate |
| Coronary artery dissection | 0.02% | Catheter-induced coronary artery dissection | Stenting |
| Air embolism | 0.02% | Operator-dependent complication; it is more common when infusion is performed through a microcatheter. It can be serious if not treated fast | Administer oxygen at 100% and wash the artery with saline serum multiple times (after making sure there is no more air). Inotropic and/or ventricular support (or both) may be needed |
| Catheter-induced spasm | 0.02% | More common in the RCA | Try to avoid nitroglycerin in the absence of flow lost. It is often a transient phenomenon |
The percentages disclosed were estimated based on 6183 procedures reported in 9 different studies. * According to the CorMicA trial, the rate of atrial fibrillation with the fastest doses infused was 6%.6 RCA, right coronary artery. Adapted with permission from Ciliberti et al.40 | |||
During the infusion of acetylcholine, sinus bradycardia, sinus arrests or episodes of atrioventricular block are common. This is often associated with too fast infusions, especially in the right coronary artery. If these complications occur, infusion should stop for a few seconds and restarted at a slower velocity. Atrial fibrillation can sometimes occur, but it often solves spontaneously; the most persistent cases often solve after the administration of amiodarone or other antiarrhythmic drugs. If bradyarrhythmias make a comeback, the test should stop immediately or be performed with a transient pacemaker in very selected cases where the test is considered indispensable.
An unwanted effect of the test is flow-limiting vasospasm that is not well-tolerated. In general, the consequences depend on the time elapsed between the occurrence of the vasospasm and the infusion of intracoronary nitrates to reverse it. The ischemia originated can cause hypotension and ventricular fibrillation that should be treated with nitroglycerin and immediate defibrillation. To stop this from going unnoticed, the patient’s blood pressure should be checked halfway into the infusion of acetylcholine, especially after the highest doses have been infused and when injected into a dominant left coronary branch. Under no circumstance a growing dose of acetylcholine should be infused if a significant or flow-limiting spasm or any other important complication have been spotted after the infusion of lower doses. Also, we should remember that, at the end of the infusion, the guide catheter still contains 2 mL of acetylcholine dilution that should be slowly pushed with a saline solution to stop it from entering the bolus with the injection of contrast. Same as it happens with any other invasive coronary procedures, and especially in this test, preloaded nitroglycerin should be available and ready to be infused. In most of the cases its infusion causes vasodilation, and fast flow recovery without needing further doses. On the other hand, atropine is a cholinergic receptor antagonist that can be used as an antidote when necessary.
Some operators perform the vasoreactivity test using the pressure guidewire inside the coronary artery as a safety measure. This brings more stability to the catheter, provides better selective infusion of dilutions, and allows us to monitor distal pressure (that can decrease in the case of flow-limiting spasm). Also, it controls the velocity of manual infusion in case of a long infusion without a pump (temperature or velocity changes are indicative that infusion is happening too fast). However, we should remember that the passage of the guidewire itself can cause vasospasm. Actually, it can simulate pseudo-spasms in tortuous arteries due to curve rectification.
INTERPRETING THE CORONARY VASOSPASM PROVOCATION TESTING
General concepts
Interpreting this test rests on 3 basic pillars:
The reproduction of the patient’s common symptoms that motivated the test. With the last dose of acetylcholine patients often experience changes of rhythm (eg, P-wave block or bradycardia) that can cause symptoms. These disorders should be distinguished from the patient’s usual angina symptoms.
The presence of changes on the ECG suggestive of ischemia, especially if accompanied by the angina symptoms that motivate the study. This assessment is often performed a few seconds after the infusion of each dose of acetylcholine. We should remember that in the presence of epicardial spasm with decreased blood flow in some of the epicardial arteries, the ST-segment does not need to be elevated or more changes on the ECG need to be present. That is so because patient’s safety is a priority at all time. Also, we should remember that, sometimes, the same injection of contrast or saline solution causes changes on the ECG. That is why serial ECGs (or collections of registries) should be performed a few seconds after the infusion of acetylcholine and before the cine coronary arteriography required (with the corresponding infusion of contrast).
The presence of angiographic coronary spasm (macrovascular) as seen on the serial registries (with cine-fluoroscopy) after every dose of acetylcholine. Spasm is defined as an obstruction with "65; 90% stenosis with respect to the diameter of the artery in this segment after the infusion of nitroglycerin. Diameter stenosis (DS) can be determined visually or using a quantitative coronary angiography. The DS is measured by obtaining the minimal luminal diameter after the dose of acetylcholine with greater vasoconstriction (MLD_ACh) with respect to the reference vessel diameter calculated after the infusion of nitroglycerin (RVD_NTG) with the following formula:
DS = 100 – [(MLD_Ach / RVD_NTG) × 100]
In practice, it is better to use the quantitative coronary angiography on the proximal segments of major arteries than in more distal segments where the reference diameter is often small and, according to the formula described above, could underestimate the DS.
Possible test results
Figure 1 shows the 4 results that can be obtained from a vasospasm provocation test performed by the infusion of intracoronary acetylcholine:
Negative test with vasodilator response (with respect to baseline). The presence of vasodilator response without symptom onset or changes on the ECG is suggestive of a normal endothelial function at epicardial level.
Negative test with vasoconstrictor response (with respect to baseline). The presence of epicardial vasoconstriction after acetylcholine without criteria of epicardial or microvascular vasospasm (defined by the lack of symptoms, changes on the ECG or significant vasoconstriction) is indicative of endothelial dysfunction, especially if vasoconstriction is confirmed after the infusion of the first few doses. Since the vasospasm provocation test has not been designed to assess the endothelial function (that requires a slower infusion rate) a certain degree of vasoconstriction is often seen with the highest dose due to the acetylcholine-induced direct stimulation of the vascular smooth muscle, which is not necessarily suggestive of epicardial endothelial dysfunction.
Positive test for epicardial spasm. The diagnosis of epicardial vasospasm requires the 3 following simultaneous findings:
Reproduction of symptoms after the infusion of acetylcholine.
Changes on the ECG suggestive of ischemia, usually in the ST-segment (whether depression or elevation > 0.1 mV). The appearance of negative U-waves has been described too.
Spasm with a "65; 90% diameter stenosis with respect to the same segment after the infusion of nitroglycerin that can be flow-limiting, focal, multisegmental or diffuse.
Positive test for microvascular spasm. Microvascular spasm has been defined as the reproduction of common angina symptoms plus the finding of changes on the ECG indicative of ischemia (basically, ST-segment depression or elevation > 0.1 mV) in the absence of coronary spasm with a "65; 90% diameter stenosis (with respect to nitroglycerin).
LEGAL ASPECTS PERTAINING TO THE USE OF INTRACORONARY ACETYLCHOLINE
The use of drugs in off-label indications different from those approved in their instructions for use and outside the clinical trial setting like intracoronary acetylcholine for diagnostic purposes requires the approval of local pharmaceutical committees. Because the Spanish Agency of Medicines and Medical Devices accepts the use of these drugs under very particular circumstances no general consensus has been achieved and local approvals are still required.
Also, in observance of Royal Decree 1015/2009 of 19 June,41 the use of a drug through a route of administration different from the one described in the drug labeling requires the provision of information as well as the patient’s written informed consent prior to its administration. For that reason, some centers also require the signing of a specific informed consent to be able to use intracoronary acetylcholine. These documents are available on the center intranet or the hospital pharmacy.
CONCLUSIONS
Figure 5 summarizes the key takeaways of this document. In conclusion, vasoreactivity testing with acetylcholine is an essential part of the assessment of patients with non-obstructive coronary artery disease and symptoms or ischemia. The result of this assessment allows us to target specific therapies and has proven effective in the routine clinical practice. Cath labs should be prepared to perform this kind of tests, and computers should be ready to use and interpret them.
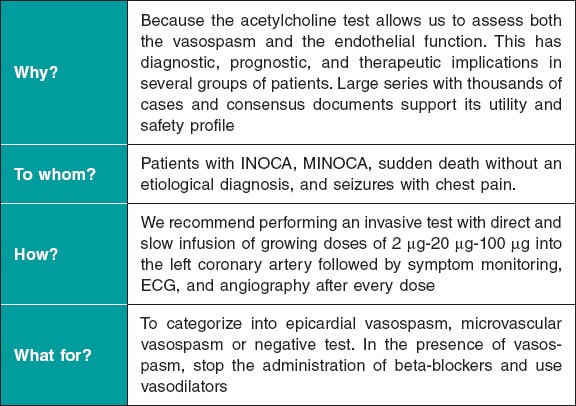
Figure 5. Key takeaways of this document. ECG, electrocardiogram; INOCA, ischemia with non-obstructive coronary arteries; MINOCA, myocardial infarction with non-obstructive coronary arteries.
FUNDING
This document had no funding whatsoever.
AUTHORS’ CONTRIBUTIONS
E. Gutiérrez and J. Gómez-Lara equally contributed to the manuscript first draft, and to the figures, and tables. The remaining authors performed a thorough revision of the paper and made comments and changes to its content and form.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation:The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177.
2. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
3. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367.
4. Aziz A, Hansen HS, Sechtem U, Prescott E, Ong P. Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. J Am Coll Cardiol. 2017;70:2349-2358.
5. Beltrame JF, Crea F, Kaski JC, et al.;Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565-2568.
6. Ford TJ, Stanley B, Good R, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina:The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841-2855.
7. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology &Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
8. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009-1086.
9. Lerman A, Burnett JC, Jr., Higano ST, McKinley LJ, Holmes DR, Jr. Longterm L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123-2128.
10. JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779-2801.
11. Sueda S, Miyoshi T, Sasaki Y, Sakaue T, Habara H, Kohno H. Safety and optimal protocol of provocation test for diagnosis of multivessel coronary spasm. Heart Vessels. 2016;31:137-142.
12. Sueda S, Kohno H, Fukuda H, et al. Induction of coronary artery spasm by two pharmacological agents:comparison between intracoronary injection of acetylcholine and ergonovine. Coron Artery Dis. 2003;14:451-457.
13. Saternos HC, Almarghalani DA, Gibson HM, et al. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics. 2018;50:1-9.
14. Lamping KG, Wess J, Cui Y, Nuno DW, Faraci FM. Muscarinic (M) receptors in coronary circulation:gene-targeted mice define the role of M2 and M3 receptors in response to acetylcholine. Arterioscler Thromb Vasc Biol. 2004;24:1253-1258.
15. Gutierrez E, Flammer AJ, Lerman LO, Elizaga J, Lerman A, Fernandez-Aviles F. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J. 2013;34:3175-3181.
16. Zeiher AM, Krause T, Schachinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345-2352.
17. Lavi S, Bae JH, Rihal CS, et al. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525-1530.
18. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899-1906.
19. Pargaonkar VS, Lee JH, Chow EKH, et al. Dose-Response Relationship Between Intracoronary Acetylcholine and Minimal Lumen Diameter in Coronary Endothelial Function Testing of Women and Men With Angina and No Obstructive Coronary Artery Disease. Circ Cardiovasc Interv. 2020;13:e008587.
20. Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary Vasomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. 2012;59:655-662.
21. Hasdai D, Gibbons RJ, Holmes DR, Jr., Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390-3395.
22. Hoshino M, Yonetsu T, Mizukami A, et al. Moderate vasomotor response to acetylcholine provocation test as an indicator of long-term prognosis. Heart Vessels. 2016;31:1943-1949.
23. Davis SF, Yeung AC, Meredith IT, et al. Early endothelial dysfunction predicts the development of transplant coronary artery disease at 1 year posttransplant. Circulation. 1996;93:457-462.
24. Gomez-Lara J, Oyarzabal L, Brugaletta S, et al. Coronary endothelial and microvascular function distal to polymer-free and endothelial cell-capturing drug-eluting stents. The randomized FUNCOMBO trial. Rev Esp Cardiol. 2021. https://doi.org/10.1016/j.rec.2021.01.007.
25. Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N. Angina pectoris. A variant form of angina pectoris;preliminary report. Am J Med. 1959;27:375-388.
26. Okumura K, Yasue H, Matsuyama K, et al. Sensitivity and specificity of intracoronary injection of acetylcholine for the induction of coronary artery spasm. J Am Coll Cardiol. 1988;12:883-888.
27. Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723-1730.
28. Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20.
29. Ford TJ, Ong P, Sechtem U, et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease:Why, How, and When. JACC Cardiovasc Interv. 2020;13:1847-1864.
30. Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046-1051.
31. Takagi Y, Yasuda S, Takahashi J, et al. Clinical implications of provocation tests for coronary artery spasm:safety, arrhythmic complications, and prognostic impact:multicentre registry study of the Japanese Coronary Spasm Association. Eur Heart J. 2013;34:258-267.
32. Widmer RJ, Samuels B, Samady H, et al. The functional assessment of patients with non-obstructive coronary artery disease:expert review from an international microcirculation working group. EuroIntervention. 2019;14:1694-1702.
33. Kim JW, Park CG, Suh SY, et al. Comparison of frequency of coronary spasm in Korean patients with versus without myocardial bridging. Am J Cardiol. 2007;100:1083-1086.
34. Ong P, Athanasiadis A, Sechtem U. Intracoronary Acetylcholine Provocation Testing for Assessment of Coronary Vasomotor Disorders. J Vis Exp. 2016;114:54295.
35. Hasdai D, Cannan CR, Mathew V, Holmes DR, Jr., Lerman A. Evaluation of patients with minimally obstructive coronary artery disease and angina. Int J Cardiol. 1996;53:203-208.
36. ENCORE Investigators. Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease:the ENCORE I Study (Evaluation of Nifedipine and Cerivastatin On Recovery of coronary Endothelial function). Circulation. 2003;107:422-428.
37. Luscher TF, Pieper M, Tendera M, et al. A randomized placebo-controlled study on the effect of nifedipine on coronary endothelial function and plaque formation in patients with coronary artery disease:the ENCORE II study. Eur Heart J. 2009;30:1590-1597.
38. Melikian N, Kearney MT, Thomas MR, De Bruyne B, Shah AM, MacCarthy PA. A simple thermodilution technique to assess coronary endothelium-dependent microvascular function in humans:validation and comparison with coronary flow reserve. Eur Heart J. 2007;28:2188-2194.
39. Diez-Delhoyo F, Gutierrez-Ibanes E, Sanz-Ruiz R, et al. Prevalence of Microvascular and Endothelial Dysfunction in the Nonculprit Territory in Patients With Acute Myocardial Infarction. Circ Cardiovasc Interv. 2019;12:e007257.
40. Ciliberti G, Seshasai SRK, Ambrosio G, Kaski JC. Safety of intracoronary provocative testing for the diagnosis of coronary artery spasm. Int J Cardiol. 2017;244:77-83.
41. Real Decreto 1015/2009, de 19 de junio, por el que se regula la disponibilidad de medicamentos en situaciones especiales. BOE núm. 174, de 20/07/2009. Available online: https://www.boe.es/eli/es/rd/2009/06/19/1015. Accessed 25 Apr 2021.
* Corresponding author: Departamento de Cardiología Intervencionista, Hospital Universitari de Bellvitge, Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
E-mail address: gomezjosep@hotmail.com (J. Gómez-Lara).
Editorials
Transcatheter aortic valve replacement for noncalcified aortic regurgitation. Where are we now?
aServicio de Cardiología, Hospital Clínico Universitario, Valladolid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain
Original articles
Editorials
Vascular closure devices: the jury is still out
aUnidad de Hemodinámica, Servicio de Cardiología, Hospital General Universitario Dr. Balmis, Instituto de Investigación Sanitaria y Biomédica de Alicante (ISABIAL), Alicante, Spain
bDepartamento de Medicina Clínica, Universidad Miguel Hernández, Alicante, Spain
Original articles
Debate
Debate: Asymptomatic severe aortic stenosis: when should we intervene?
The clinician’s perspective
Servicio de Cardiología, Hospital Ramón y Cajal, Madrid, Spain
The interventional cardiologist’s perspective
Unidad de Cardiología Intervencionista, Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo, Vigo, Pontevedra, Spain