ABSTRACT
Introduction and objectives: Rapid ventricular pacing reduces cardiac output by providing stability during transcatheter aortic valve implantation (TAVI). Our objective is to assess the efficacy and safety profile of left ventricular pacing through the high-support guidewire used for implantation and a guidewire located in the right atrium (RA) functioning as an anode.
Methods: Left ventricular pacing is performed by connecting the external end of a Safari2 pre-shaped guidewire located in the left ventricle to the cathode of a temporary pacemaker, and the anode to the body of an Emerald guidewire inserted into the RA using a diagnostic Judkins Right catheter (via ultrasound-guided femoral venous access). Pacemaker was programmed with maximum output (20 V) and null sensitivity.
Results: A total of 62 selected patients (median 79.4 ± 6.5 years old) underwent transfemoral TAVI using the pacing technique described (25 patients the SAPIEN 3 Ultra; 13 the Navitor, 9 the ACURATE neo2, 14 the Evolut PRO+, and 1 patient the Myvalve). Procedure was successful in all cases (there was 1 capture failure due to pacemaker programming). Two patients required a temporary and permanent pacemaker due to high-grade atrioventricular block. No vascular complications from venous access were documented, not even from the RA guidewire. Procedural time did not increase significantly, and the median length of stay after implantation was 2 days.
Conclusions: In our series, left ventricular pacing using the RA-positioned wire as the anode proved to be effective and safe without increasing procedural time significantly. This procedure also provides the advantage of being able to use the central venous access for possible emergency temporary pacemaker implantation.
Keywords: Aortic stenosis. Transcatheter aortic valve implantation. Left ventricular pacing. Right atrium. Femoral venous access.
RESUMEN
Introducción y objetivos: La estimulación ventricular rápida reduce el gasto cardiaco, proporcionando estabilidad durante el implante percutáneo de válvula aórtica (TAVI). Nuestro objetivo fue evaluar la eficacia y la seguridad de la estimulación ventricular izquierda a través de la guía de alto soporte utilizada para el implante y una guía situada en la aurícula derecha (AD) que actúa como ánodo.
Métodos: La estimulación ventricular izquierda se realiza conectando el extremo externo de una guía Safari2 preformada situada en el ventrículo izquierdo al cátodo de un marcapasos temporal, y el ánodo al cuerpo de una guía Emerald insertada en la aurícula mediante un catéter Judkins Right diagnóstico a través de un acceso venoso femoral (punción ecoguiada). El marcapasos se programa con salida máxima (20 V) y sensibilidad anulada.
Resultados: Se realizó TAVI transfemoral a 62 pacientes seleccionados (mediana de edad: 79,4 ± 6,5 años) utilizando la técnica de estimulación descrita (25 SAPIEN 3 Ultra, 13 Navitor, 9 ACURATE neo2, 14 Evolut PRO+ y 1 Myvalve), con éxito en todos los casos (hubo 1 fallo de captura atribuido a la programación del generador del marcapasos). Dos pacientes necesitaron marcapasos transitorio y definitivo posterior por bloqueo auriculoventricular completo durante el procedimiento. No se documentaron complicaciones vasculares derivadas del acceso venoso ni del posicionamiento de la guía en la AD. No aumentaron de manera significativa el tiempo del procedimiento ni la fluoroscopia. La mediana de estancia hospitalaria tras el implante fue de 2 días.
Conclusiones: En nuestra serie, la estimulación ventricular izquierda utilizando como ánodo la guía situada en la AD ha demostrado ser una técnica eficaz y segura, sin aumentar significativamente el tiempo de procedimiento, y además aporta la ventaja de disponer de acceso venoso central para un posible marcapasos transitorio urgente.
Palabras clave: Estenosis aórtica. Implante percutáneo de válvula aórtica. Estimulación ventricular izquierda. Aurícula derecha. Acceso venoso femoral.
Abbreviations AVB: atrioventricular block. JR: Judkins Right catheter. RA: right atrium. RBBB: right bundle branch block. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Twenty years have passed since A. Cribier performed the first transcatheter aortic valve implantation (TAVI) in humans.1 Since 2002 and up until today, the studies published have demonstrated the non-inferiority of TAVI compared to surgical aortic valve replacement in high- (PARTNER 1A),2 moderate- (PARTNER 2 and SURTAVI)3,4 and low-surgical risk patients (PARTNER 3 and Evolut Low Risk).5,6 Also, the PARTNER 3 demonstrated the superiority of transfemoral TAVI with the balloon-expandable SAPIEN 3 val v e (Edwards Lifesciences, United States). It all has changed our routine clinical practice, and the European guidelines on the management of valvular heart disease7 published back in 2021 recommend transfemoral TAVI like the treatment of choice for patients with severe aortic stenosis > 75 years regardless of their surgical risk involved.
Over the past few years, the implantation technique has become easier thanks to procedural standardization, the operators’ experience, the technological evolution of the devices, and the tendency to perform minimalist approaches,8 thus reducing complications and allowing faster patient recovery times.
Rapid ventricular pacing necessary to reduce the cardiac output and promote stability during balloon valvuloplasty, valve deployment or postdilatation have traditionally been performed through temporary pacemaker implantation into the right ventricle (RV). A way to simplify the procedure and reduce cost that has proven safe and effective is to perform pacing through a high-support guidewire located at left ventricular (LV) level9 that is connected to the cathode (negative electrode) of the generator of temporary pacemaker. In most cases described in the medical literature available, the anode (positive electrode) connects to a needle inside the patient’s skin or subcutaneous cellular tissue.
This study is a different take on the left ventricular pacing technique where the guidewire inserted into the right atrium (RA) acts as an anode. Its efficacy and safety profile, and advantages compared to conventional traditional techniques available will be assessed here.
METHODS
This is single-center, prospective, and observational registry of patients with severe aortic valvular heart disease treated with transfemoral TAVI from November 2021 through September 2022. In patients with baseline conduction disorders involving a higher risk of high-degree atrioventricular block (AVB) during or after the procedure (complete right bundle branch block, selected cases of bifascicular block or bradycardia left to the operator’s criterion), the pacing method used was temporary pacemaker implantation into the RV via jugular vein at the beginning of the procedure. In patients with low risk of AVB regardless of the type of valve implanted (balloon-expandable or self-expanding), rapid ventricular pacing was performed using a high-support guidewire of LV location. The use of a needle inside the subcutaneous cellular tissue or a guidewire into the RA as the anode was left to the operator’s criterion. In carriers of definitive pacemakers, overpacing was performed by externally programming the pacemaker (figure 1).
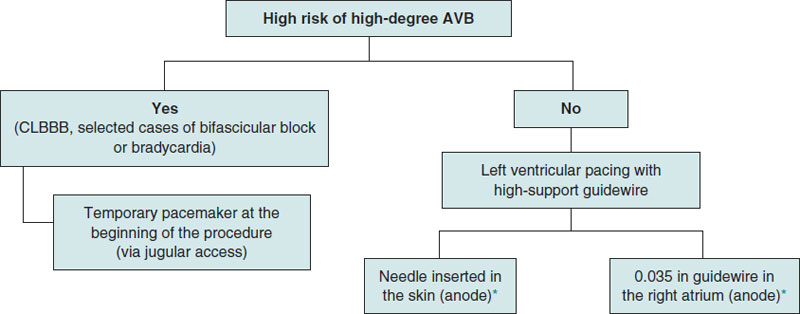
Figure 1. Protocol to select the type of pacing that should be applied during transcatheter aortic valve implantation. AVB, atrioventricular block; CLBBB, complete left bundle branch block.
* Left to the treating operator’s criterion.
Description of the technique
After crossing the aortic valve with a straight guidewire and a diagnostic AL-1 catheter, the former is exchanged for a high-support Safari2 pre-shaped guidewire (Boston Scientific, United States) that is placed in the LV (of XS or S size based on the ventricular size). This guidewire is routinely used in our center for valve implantation purposes. The guidewire external border connects to the negative electrode (cathode) of the generator of the temporary pacemaker using crocodile clips. On the other hand, the anode (positive electrode) of the generator of the temporary pacemaker connects the same way to the body of a 0.035 in guidewire inserted into the RA via femoral venous access (an ultrasound-guided puncture is performed in all cases followed bya femoral 6-Fr introducer sheath at this level).
The 0.035 in guidewire used is the Emerald (Cordis, Switzerland) with atraumatic J-shapep tip in its distal border—common in our cath lab—for catheter exchange purposes. Across its entire trajectory it is covered by a diagnostic Judkins Right (JR) catheter that acts as an electric insulator during pacing to avoid damaging the vascular structures that run through the guidewire and conduct the electrical charge towards the region of interest except for the guidewire distal border that should remain in close contact with the walls of the RA (figure 2).

Figure 2. Angiographic imaging of ventricular overpacing. JR catherer (A, black arrow), and 0.035 in guidewire (A, blue arrow) in the right atrium performing ventricular overpacing during valvuloplasty (A) balloon-expandable valve implantation (B), and postdilatation of a self-expanding valve (C).
This guidewire could also be inserted via jugular venous access. However, in our own experience, femoral vein cannulation is a safe, quick, and easy technique interventional cardiologists have become more familiar with.
Once these connections have been made, the generator of the pacemaker is programmed with the maximum energy output allowed (20-25 V) and cancelled sensitivity. Proper capture is, then, checked. Also, when performing rapid pacing, arterial pressure falls < 50 mmHg. Finally, overpacing is performed at 120 bpm to 180 bpm depending on each case. We should mention that inadequate positioning of the guidewire into the RA can lead to failed captures, which is why checking its location prior to pacing is advised.
Criteria for temporary pacemaker implantation
In cases when the patient develops high-degree AVB—both persistent and transient—during the procedure, a temporary pacemaker is implanted at the cath lab using the femoral venous access previously cannulated).10 In cases of complete AVB without ventricular escape rhythm at < 30 bmp or with a higher escape rhythm but poor hemodynamic tolerance, left ventricular pacing is maintained while the high-support guidewire remains in the LV and a needle is inserted in the skin acting as an anode until an electrocatheter of temporary pacemaker is implanted into the RV via femoral access. The femoral introducer sheath is maintained in cases of CLBBB (complete left bundle branch block) with de novo QRS complex > 150 ms or transient alternating bundle-branch block. Otherwise, it is removed when the procedure has been completed. Also, mechanical compression is performed at this level (figure 3).
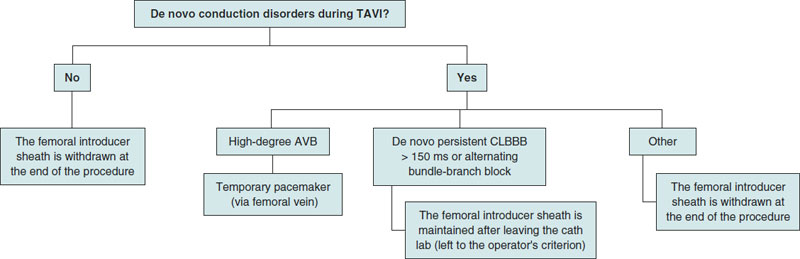
Figure 3. Criteria for temporary pacemaker implantation during the procedure. AVB, atrioventricular block; CLBBB, complete left bundle Branch block; TAVI, transcatheter aortic valve implantation.
After TAVI, the patient is admitted to the intermediate coronary care unit where cardiac rhythm is monitored for, at least, 24 hours following the 2021 ESC guidelines recommendations on cardiac pacing regarding cardiac monitorization, HV interval measurement or definitive pacemaker implantation.11 At our center, there is an X-ray room in the cardiology ward. Therefore, if temporary pacemaker implantation is required, it is swiftly implanted by the cardiology at the intermediate coronary care unit or the cardiologist on call.
Statistical analysis
Standard descriptive statistics was used for the patients’ baseline and procedural characteristics, and clinical results. Continuous variables are expressed as mean ± standard deviation or median ± interquartile range based on the sample normal distribution. Categorical variables are expressed as percentages. All statistical analyses were performed using the statistical software package SPSS V25 (IBM, United States).
RESULTS
A total of 130 patients were treated with TAVI during the time included in this analysis. Rapid ventricular pacing was performed in 62 cases (58 severe aortic stenoses and 4 pure severe aortic failures) using the high-support guidewire located at the LV and the 0.035 in guidewire located in the RA. In 36 patients a temporary pacemaker was implanted from the beginning of the procedure and in the remaining ones (29 patients), left ventricular pacing was performed using the needle inserted into the patient’s skin as the anode.
The baseline characteristics of the cohort of patients paced with the guidewire in the RA, and the procedural ones are shown on table 1. The pacing technique described has been used with balloon-expandable (42%) and self-expanding valves (58%). A total of 8 valve-in-valve procedures (12.9%) were performed while the patient’s anatomy (aortic annulus size, horizontal aorta, risk of coronary obstruction, valvular calcification, pure aortic failure…) turned out to be the main determinant of the type of valve selected.
Table 1. Demographic, clinical, and procedural characteristics of the cohort
| Baseline characteristics | |
|---|---|
| Age, years | 79.4 ± 6.5 |
| Sex (men), % | 56.3 |
| Cardiovascular risk factors, % | Arterial hypertension, 87 |
| Diabetes mellitus type 2, 44.8 | |
| Dyslipidemia, 58.1 | |
| Smoking, 24 | |
| Mean BMI, 29.7 ± 5.4 kg/m2 | |
| Chronic ischemic heart disease, % | 41.9 |
| STS score, % | 4.7 ± 3.97 |
| Baseline conduction disorders, n | |
| CRBBB | 0 |
| IRBBB | 1 |
| LASB | 1 |
| LPSB | 0 |
| CLBBB | 3 |
| First-degree AVB | 6 |
| Echocardiographic and CT data | |
| Valvular area pre-TAVI, cm2 | 0.7 ± 0.2 |
| Mean gradient pre-TAVI, mmHg | 46 ± 14 |
| Mean gradient of the annulus on the CT scan, mm2 | 454.65 |
| Perimeter, cm | 77.6 |
| Severe valvular calcification on the CT scan | 31 patients |
| Procedural data | |
| Emergency procedures, n | 2 (cardiogenic shock) |
| Conscious sedation + local anesthesia, n | 60 patients |
| General anesthesia, n | 2 (emergency procedures) |
| Predilatation, n | 26 (mean balloon, 22.3 ± 2.8 mm) |
| Self-expanding valves | 13 Navitor |
| 14 Evolut PRO+ | |
| 9 ACURATE neo2 | |
| Balloon-expandable valves | SAPIEN 3 Ultra 25 |
| 1 Myvalve | |
| Postdilatation, n | 14 (mean balloon, 23 ± 2.5 mm) |
| X-ray time, min | 19 ± 3 |
| Procedural time, min | 47 ± 10 |
| Type of closure, % | Collagen-based MANTA vascular closure device, 98.4 |
| Proglide + Angio-Seal, 1.64 | |
| Procedure-related CLBBB, n | 9 transient (2 required definitive pacing)* |
| 6 persistent (without an indication for definitive pacing) | |
| Complete posterior AVB, n | |
| Intraoperative | 2 |
| In-hospital | 1 (at 24 hours)* |
| Outpatient | 1 (5 days after discharge)* |
| Postoperative alternating bundle-branch block, n | 0 |
| Postoperative first-degree AVB > 240 ms, n | 1 |
| Immediate procedural success, % | 95.2% |
| Procedural success at 30 days, % | 93.5% |
AVB, atrioventricular block; BMI, body mass index; CLBBB, complete left bundle branch block; CRBBB, complete right bundle branch block; CT, computed tomography scan; DM2, diabetes mellitus type 2; ILBBB, incomplete left bundle branch block; LASB, left anterior subdivision block; LPSB, left posterior subdivision block; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation. | |
Capture failed in 1 patient (although suspicion abounds that the cause was the programming of the generator of the pacemaker) without final repercussion in the position of the valve being successful in the remaining cases. We should mention the optimal behavior of the technique in balloon-expandable valves where failed capture could immediately jeopardize the procedure. In our complete series of TAVI, the overall percentage of failed captures when the patient’s skin was used as the anode was 2.7% and 0.8% in cases with temporary pacemaker implantation.
Conduction disorders after transcatheter aortic valve implantation
Complete AVB was described in 2 patients during the procedure (1 Evolut PRO+ [Medtronic, United States], and 1 Navitor [Abbott, United States]). One of them had a baseline electrocardiogram with a narrow QRS complex (100 ms), and an image of incomplete right bundle branch block with a complete AVB after the valvuloplasty. The second patient showed no conduction disorders at baseline (PR, 120 ms; QRS, 90 ms), and a complete AVB after valvular deployment. Left ventricular pacing was performed in both with the Safari2 pre-shaped guidewire (using a needle inserted into the subcutaneous cellular tissue connected to the positive electrode of the generator of the pacemaker) until the insertion of an electrocatheter into the RV via femoral venous access previously cannulated, without complications to eventually implant a definitive pacemaker 24 hours later due to persistent high-degree AVB.
A total of 24.2% of the cases (15 patients) developed CLBBB after valve implantation. In 9 patients (14.5%), bundle branch block was temporary and resolved at the cath lab or a few hours after the procedure. However, one of them developed a complete AVB 24 hours after the procedure (seen during monitorization with telemetry), and another one was readmitted 5 days after valve implantation due to the presence of a symptomatic complete AVB (recurrent syncopes), which is why, in both cases, a definitive pacemaker was implanted (both patients with self-expanding valves). In the remaining cases, the CLBBB was persistent (12.3%): in 1 patient, the width of the QRS complex narrowed down from 130 ms to 120 ms during admission (4 days of monitorization), 3 patients had previously been treated with a valve-in-valve procedure and, for 5 days of admission and monitorization, they kept the same width of the QRS complex without any other conduction disorders being reported. Also, in 2 patients the HV interval was measured due to a persistent QRS complex of 140 ms and 150 ms (HV interval, 55 mm and 58 ms, respectively). Therefore, they were all discharged from the hospital without the need for definitive pacing while none of them required definitive pacemaker implantation at follow-up. We should mention that, in this cohort of patients, balloon-expandable valves had a rate of definitive pacemaker implantation of 0%.
In the 3 patients with baseline CLBBB (2 of them with first-degree AVB) no changes to the baseline electrocardiograms were seen or conduction disorders during monitorization were reported. No definitive pacing has been required at follow-up either.
The low rate of pacemaker implantation in this cohort (6.5% within the first 30 days after TAVI) despite the fact that 58% of the devices were self-expanding valves is mainly attributed to patient selection since, like we already said, in cases with baseline conduction disorders involving a high risk of high-degree AVB after TAVI, pacing is performed through temporary pacemaker implantation since the beginning of the procedure. In our entire series of patients, the rate of pacemaker implantation within the first 30 days after TAVI over the past year was 15.5% (being the rate of self-expanding valves implanted over the past 12 months, 69%).
Procedural success, and major adverse cardiovascular events
The rates of immediate procedural success (according to the VARC-3 standard definition),12 30-day procedural success, in-hospital mortality, and cardiovascular and 30-day mortality since discharge were 95.2%, 93.5%, 3.2% (2 patients), and 0%, respectively. It was necessary to implant a second valve in 1 patient due to the supra-annular position of the first valve ( ACURATE neo2 [Boston Scientific, United States] and then a SAPIEN 3 Ultra. In the emergency procedures performed the immediate and 30-day success rates were 100%.
No neurological adverse events were reported in this cohort at 30 days (in 2 cases a Sentinel device (Boston Scientific, United States) was used for cerebral protection) or coronary occlusions after TAVI. The rate of grade ≥ III major aortic regurgitation after TAVI was 0%.
No femoral venous access-related complications or due to the position of the guidewire into the RA were reported. In 98.3% of the cases, the femoral artery was closed with a collagen -based MANTA vascular closure device . A covered stent was required in 2 patients due to failed device closure. On the other hand, acute arterial ischemia occurred in 1 patient with severe peripheral arteriopathy.
The mean x-ray and procedural times were 19 ± 3 min and 48 ± 10 min, respectively without significant differences among the 3 types of pacing described. The median length of stay after TAVI was 2 days following all cases a minimalist approach.
Results regarding the procedure immediate success and mortality are similar with the 3 pacing techniques mentioned without higher rates of valve embolization, need for a second valve or significant aortic regurgitation after the procedure in cases where left ventricular pacing was performed using the guidewire located at the RA as the anode. In our overall series of TAVI, the rate of cardiac tamponade due to RV perforation by the electrocatheter insertion for temporary pacemaker implantation was 1.9%.
DISCUSSION
Rapid ventricular pacing reduces the preserved cardiac output, thus providing the necessary stability for valve deployment. Failed ventricular captures during overpacing is associated with a risk of valve embolization or malapposition with potentially devastating consequencies, which is why it is essential to achieve effective overpacing.
Traditionally, ventricular pacing has been performed by inserting a temporary pacemaker into the RV. The standard electrocatheter used for transient pacing has a rigid electrode in its distal end that increases the risk of myocardial perforation. There are other electrodes more commonly used today during TAVI that come with a small balloon in their distal border and are less traumatic. However, since they are softer and more flexible, their implantation is often more challenging and their position less stable, which increases procedural and fluoroscopy times, and the risk of failed capture during overpacing.9
Back in 2007, ventricular pacing was described for the first time through a guidewire of LV location in a series of pediatric patients with congenital severe aortic stenosis.13 It proved to be a safe and effective technique with a lower rate of vascular complications, shorter procedural times, and less expensive compared to the systematic implantation of a temporary pacemaker electrode.
Back in 2019, the EASY TAVI was published.9 It was the first randomized clinical trial to compare the 2 aforementioned pacing techniques that proved that left ventricular pacing (using, in all cases, a needle inserted into the patient’s skin as the anode) had a similar efficacy, simplified the procedure, and reduced time, fluoroscopy times, costs, and complications (with a higher rate of cardiac tamponade due to RV perforation by the pacemaker catheter). However, the study was only conducted in patients with balloon-expandable valves ( SAPIEN 3) in whom the risk of high-degree AVB is lower compared to self-expanding valves.
At our center, the rate of self-expanding valve implantation is high (69% over the past year), which is associated with a higher risk of high-degree AVB and, therefore, definitive pacemaker implantation. Therefore, left ventricular pacing using a guidewire placed in the RA as the anode (positive electrode) is a very effective technique to have a vascular access available since the beginning of the procedure. Therefore, in case of high-degree AVB, temporary pacemaker implantation is often performed quickly to avoid the systematic implantation of an electrocatherer into the RV, thus reducing procedural costs and the rate of complications. On the other hand, in balloon-expandable valves where failed captures during overpacing could jeopardize the immediate success of the procedure, this technique has worked optimally.
We should mention the fact that temporary pacemaker implantation since the beginning of the procedure conditions a lower threshold to keep after leaving the cath lab, thus increasing the risk of infectious, vascular, thromboembolic or cardiac complications and delaying the start of patient mobilization. In cases of left ventricular pacing and RA guidewire, the temporary pacemaker is only kept after leaving the cath lab in the 2 patients with high-degree AVB during implantation. However, in the cohort of patients with temporary pacemaker implantation right from the start, 88.9% (32 patients) leave the cath lab with a temporary pacemaker on that was kept for nearly 24 hours. Finally, 37% of these patients (13) had an indication for definitive pacing.
Regarding the use of femoral venous access, we should mention that it comes with some disadvantages14 regarding the jugular vein. Basically, it’s a less direct access towards the RV, less aseptic, and limits the patient’s mobility until definitive pacemaker implantation. However, the cannulation of the femoral vein is an easy-to-do, safe, and fast technique for interventional cardiologists who are often not that used to jugular venous access that is often cannulated by the assisting anesthesiologist. Less experienced operators can have issues too. In our case, femoral venous puncture was ultrasound-guided in all the cases, and the rate of complications at this level was 0%, being the introducer sheath withdrawn at the end of the procedure in all the patients who did not require a temporary pacemaker or a central venous catheter. In cases that required definitive pacing, the pacemaker was implanted after 24 hours, which reduces complications and minimizes the patient’s mean length of stay.
There is only 1 single-center, observational study in the medical literature available15 with prospective recruitment of patients and retrospective analysis where left ventricular pacing was performed with a high-support guidewire for valve implantation (Safari) plus a standard guidewire inserted into the inferior vena cava (without introducer sheath) in 226 non-selected patients treated with TAVI from March 2017 through September 2018 (27.4%, Core V alve; 16.4%, SAPIEN ; 56.2%, ACURATE neo). The efficacy of pacing was 99.1% (2 patients required temporary pacemaker implantation due to failed captures with the guidewire). Additionally, in 7.6% of the patients a temporary pacemaker had to be implanted due to conduction disorders during the procedure. Vascular complications occurred in 2.7% of the patients, and the rate of definitive pacemaker implantation was 14%.
One of the main differences with our study is the previous selection of cases at our center being patients with a low baseline risk of developing AVB after TAVI those who benefit the most from pacing with a high-support guidewire into the LV. On the contrary, temporary pacemaker implantation should be considered right from the beginning of the procedure in the presence of baseline conduction disorders that increase the risk of high-degree AVB (mainly complete right bundle branch block). Since there are higher chances of definitive pacing, jugular venous access can be used in these patients for the advantages already mentioned. On the other hand, the technical differences are the insertion of the guidewire into the RA instead of the inferior vena cava—which allows us to check its position at all time to minimize possible failed captures—the fact that the guidewire is covered with a diagnostic catheter that works as an insolator, use a femoral introducer sheath to speed up electrocatheter implantation into the RV, if necessary, and the availability of a central venous catheter during the procedure.
In conclusion, our study proves the efficacy and safety of the pacing technique described in our population of patients treated with transfemoral TAVI with satisfactory results, fewer temporary pacemakers implanted over the past year, and lower procedural cost without complications associated with femoral venous access or guidewire placement into the RA.
Limitations
The study main limitation is that it is an observational, single-center study with a small sample of patients describing all the results obtained since the technique was first used in our center, initially performed by 2 operators and in very selected cases. However, currently, the pacing technique described has been included in the routine interventional clinical practice of our cath labs.
CONCLUSIONS
In our own experience, left ventricular pacing using the needle placed in the RA as the anode has proven a very safe and effective technique in patients with low risk of AVB due to TAVI without increasing procedural time significantly. This technique cuts the costs associated with the systematic use temporary pacemaker. Also, it provides a venous access fully available since the start of the procedure for possible emergency temporary pacemaker implantation.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All the authors contributed to the study design, performed a critical review of the manuscript, gave their final approval, and are fully responsible for all aspects of the study guaranteeing both its integrity and accuracy.
WHAT IS KNOWN ABOUT THE TOPIC?
- Rapid ventricular pacing is necessary to reduce the cardiac output and provide stability during TAVI. Therefore, traditionally, a transvenous temporary pacemaker has been placed in the RV. However, its systematic use increases procedural risk, fluoroscopy time, and above all, total cost. Therefore, left ventricular pacing performed through a high-support guidewire used for implantation simplifies the procedure, has proven safe, and has a similar efficacy.
WHAT DOES THIS STUDY ADD?
- This article showed a change to the left ventricular pacing technique in which a high-support guidewire located at the LV was used (connected to the negative electrode of a temporary pacemaker) plus a guidewire placed in the RA (connected to the positive electrode). In our own experience, it is a safe and effective technique without significant differences in procedural or fluoroscopy time with respect to the traditional way of guidewire-driven LV pacing. Also, it provides us with a venous access fully available ritgh from the start of the procedure to facilitate quick electrocatheter implantation into the RV in cases of high-degree AVB.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis First Human Case Description. Circulation. 2002;106:3006-3008.
2. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
3. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk patients. N Engl J Med. 2016;374:1609-1620.
4. Reardon MJ, Van Mieghem NM, Pompa JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk patients. N Engl J Med. 2017;376:1321-1331.
5. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705.
6. Pompa JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706-1715.
7. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
8. Lauck SB, Wood DA, Baumbusch J, et al. Vancouver Transcatheter Aortic Valve Replacement Clinical Pathway: Minimalist Approach, Standardized Care, and Discharge Criteria to Reduce Length of Stay. Circ Cardiovasc Qual Outcomes. 2016;9:312-321.
9. Faurie B, Souteyrand G, Staat P, et al. Left Ventricular Rapid Pacing Via the Valve Delivery Guidewire in Transcathter Aortic Valve Replacment. JACC Cardiovasc Interv. 2018;11:1663-1665.
10. Jorgensen TH, De Backer O, Gerds TA, et al. Inmediate Post-Proceedural 12-Lead Electrocardiography as Predictor of Late Conduction Defects After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018;11:1509-1518.
11. Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:427-3520.
12. Généreux P, Piazza N, Alu MC, et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021;77:2717-2746.
13. Karagöz T, Aypar E, Erdogan I, et al. Congenital aortic stenosis: a novel technique for ventricular pacing during valvuloplasty. Catheter Cardiovasc Interv. 2008;72:527-530.
14. Chun KJ, Gwag HB, Hwang JK, et al. Is transjugular insertion of a temporary pacemaker a safe and effective approach? PLoS One. 2020;15:e0233129.
15. Scarsini R, Kotronias RA, De Maria GL, et al. Routine Left Ventricular Pacing for Patients Undergoing Transcatheter Aortic Valve Replacement. Structural Heart. 2019;3:478-482.











