To the Editor,
This is the case of a 57-year-old man with a past medical history of thoracic radiation in his adolescence in the context of Hodgkin’s lymphoma. The patient also has unclassified interstitial lung disease and endothoracic goitre with tracheal displacement creating a difficult airway. Back in 2014, the patient was diagnosed with chronic coronary syndrome and complete percutaneous revascularization of right coronary artery was achieved. The echocardiograms revealed the presence of severe mitral valve stenosis due to valve calcification without signs of rheumatic disease, preserved systolic function, and severe pulmonary hypertension.
The patient started showing progressive signs of heart failure, and aortic valve replacement was indicated. Given the evidence of porcelain aorta, transcatheter aortic valve implantation (TAVI) was decided using a 23 mm Edwards SAPIEN valve (Edwards Lifesciences, United States) under deep sedation via femoral access. No complications were reported at discharge.
Follow-up in the valve clinic showed progressive worsening of dyspnea (New York Heart Association [NYHA] class III). Additional tests confirmed the progression of mitral stenosis. The transthoracic (TTE) and transesophageal (TEE) echocardiography confirmed the presence of extensive mitral annular calcification (MAC) causing severe mitral stenosis (mean gradient, 15 mmHg) without regurgitation, mild right ventricular dilatation with preserved function, and estimated systolic pulmonary artery pressure > 60 mmHg. The valve was working properly.
The case was reassessed, and the possibility of transcatheter biological aortic valve implantation in the mitral position was considered given the surgical and anesthetic risks involved.
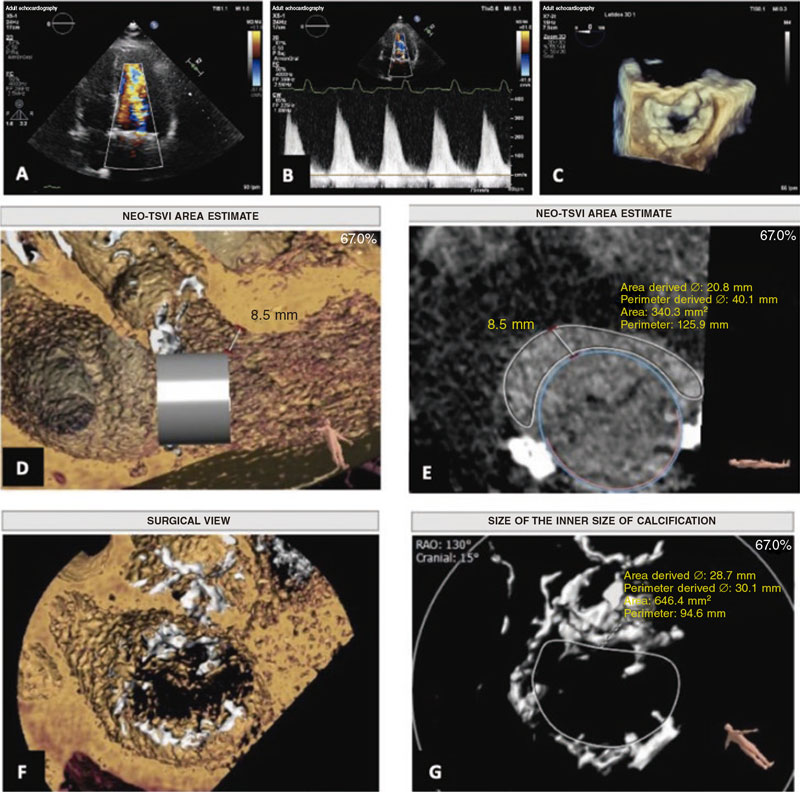
The feasibility study of the procedure assessed using the 3mensio Structural Heart system (Pie Medical Imaging, The Netherlands) revealed the presence of mitral annular calcification with a circumferential extension of 298°, no calcification in the medial commissure, anteroposterior and intercommissural diameters of 24.5 mm and 33 mm, respectively, and a inner area of 646 mm2. These measurements were considered eligible for the 29 mm Edwards SAPIEN valve implantation.
In the simulation, estimating the neo-left ventricular outflow tract (LVOT)1 area (340 mm2) was particularly relevant, considered low risk for LVOT obstruction (figure 1). The results and the echocardiographic images obtained proved the case eligible for valve implantation in mitral annular calcification, also known as valve-in-MAC1 via transfemoral access. The patient was informed of the high complexity and morbidity and mortality associated with the procedure,2,3 with long-term results still unknown to this date.
Figure 1. Previous study of transcatheter valve implantation in mitral annular calcification.A: 4-chamber apical echocardiogram with color Doppler showing acceleration of diastolic flow through the mitral valve.B: continuous wave Doppler.C: view of the mitral annulus using transesophageal echocardiography.D andE: estimate of the diameter and area of the neo-left ventricular outflow tract using the 3mensio system.F andG: view and measurements of mitral annular calcification using the 3mensio system. NEO-LVOT, neo-left ventricular outflow tract.
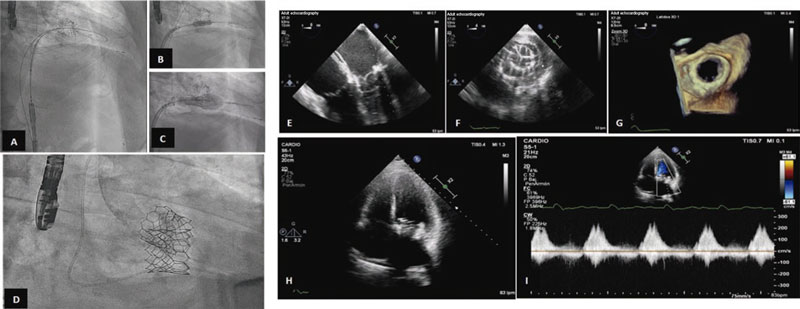
The procedure was performed back in March 2022 with the patient presenting to the cath lab with clinical data of heart failure. Under general anesthesia and intubation with a fiberoptic bronchoscope, a conventional TEE probe was advanced uneventfully. Under TEE and fluoroscopy guidance, a transseptal puncture was performed via venous femoral. Afterwards, the left atrium was accessed with an Agilis Nxt catheter (Abbott Laboratories, United States), and the mitral valve was crossed using a conventional J-tip guidewire. With back-up from a 7-Fr coronary guide catheter, 2 Safari high-support guidewires (Boston Scientific, United States) were placed into the left ventricle. The interatrial septum was dilated using a 12 mm angioplasty balloon, and a 29 mm Edwards SAPIEN 3 valve was advanced until it was eventually placed inside the mitral orifice, and then properly aligned.
Once its proper position was confirmed, the valve was implanted under pacemaker stimulation that remained in the desired position without paravalvular leak (figure 2). The immediate postoperative mean diastolic mitral gradient was 8 mmHg. The main complication was complete atrioventricular block that persisted after the procedure. The patient was uneventfully extubated in the cath lab.
Figure 2. Valve implantation and outcomes.A to D: fluoroscopy showing the previous transcatheter aortic valve implantation, and the process of advancement, placement, and expansion of the aortic valve implanted in the mitral position with Safari high-support guidewire implantation into the left ventricle. E: mid-esophageal, 4-chamber view at 0 degrees of transesophageal echocardiography (TEE) imaging showing the already implanted biological valve in the mitral position.F: transgastric cross-sectional TEE image at mitral valve level showing the biological valve implanted in the mitral position.G: 3D TEE reconstruction of the mitral view showing the valve implanted in valve opening mode.H andI: apical 4-chamber transthoracic echocardiography showing the mitral valve implanted and diastolic mitral flow with a mean gradient of 8 mmHg.
The postoperative period was uneventful, and the signs of congestion seen at admission were resolved. Episodes or paroxysmal atrialfibrillation episodes were reported, and a definitive DDD pacemakerwas implanted due to persistent atrioventricular block. The patient was discharged after staying 20 days at the hospital on anticoagulant therapy with acenocoumarol.
Periodic clinical follow-up was performed 2, 6, and 9 months after the procedure. The patient remained in NYHA functional class I with a mean diastolic mitral gradient of 8 mmHg. Pulmonary artery systolic pressure dropped significantly, and no LVOT obstruction was seen.
The use of valve-in-MAC via transfemoral venous access is a novel technique in Spain that has not been widely explored until now. However, in this case, the 9-month follow-up outcomes were good regarding quality of life and improvement of hemodynamic parameters.
A clinical trial (NCT03539458) currently in the pipeline is analyzing the use of valves specifically designed for transcatheter mitral valve implantation in patients with mitral annular calcification and severe mitral regurgitation.4 However, no clinical trials on the management of patients with mitral annular calcification and severe mitral stenosis have ever been conducted. In a series of cases including 23 procedures,3 a 17% mortality rate and a high rate of complications at 30 days were seen in patients undergoing a similar transcatheter procedure as the one described in this case report. Therefore, it is necessary to move forward in the design of techniques that may result in good short- and long-term technical and clinical outcomes for this specific group of patients.
The patient gave his verbal consent for the publication of this study.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All the authors contributed equally to the drafting and review process of the manuscript.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Serban R, Redwood S, Prendergast B, Rajani R. Real-time image integration for transcatheter mitral valve replacement in mitral annular calcification. J Thorac Cardiovasc Surg. 2019;157:e135-139.
2. Guerrero M, Urena M, Himbert D, et al. 1-year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol. 2018;71:1841-1853.
3. Al-Hijji MA, El Hajj S, El Sabbagh A, et al. Temporal outcomes of transcatheter mitral valve replacement in native mitral valve disease with annular calcification. Catheter Cardiovasc Interv. 2021;98:E602-609.
4. Feasibility Study of the Tendyne Mitral Valve System in Mitral Annular Calcification [Internet]. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03539458. Accessed 1 Feb 2023.