ABSTRACT
Introduction and objectives: Transfemoral access is globally accepted as the preferential access route for transcatheter aortic valve implantation (TAVI). However, in up to 15% of the patients, this access is considered inadequate. Considering the various alternatives available, the fully percutaneous access routes have been chosen preferentially. This analysis aims to compare outcomes and complications of 3 alternative access routes for transfemoral, trans-subclavian and transcaval TAVI.
Methods: Retrospective analysis of patients referred for TAVI using transfemoral, trans-subclavian, and transcaval accesses in a single tertiary center from 2008 through 2021. The primary endpoints were 30-day and 1-year all-cause mortality rates. The secondary endpoints were technical success, residual moderate-to-severe paravalvular leak, major vascular complication, 30-day stroke, 30-day Valve Academic Research Consortium-2 (VARC-2) major bleeding, and 30-day acute kidney injury (AKIN criteria 2 or 3).
Results: A total of 642 TAVIs were performed (601 transfemoral, 24 trans-subclavian, and 10 transcaval). A total of 7 patients treated via transapical access were excluded. As expected, baseline comorbidities like left ventricular dysfunction, coronary artery disease, atrial fibrillation, chronic kidney disease, and previous stroke were more frequent in the non-femoral groups. The 1-year and 30-day all-cause mortality rates were higher in the non-transfemoral population (HR, 2.88 and HR, 3.53, respectively). The rates of 30-day stroke and acute kidney injury (AKIN 2 or 3) were also significantly lower in transfemoral patients, but similar between trans-subclavian and transcaval patients. The rates of 30-day major bleeding showed a statistically significant tendency towards lower rates in the transfemoral group. The rates of technical success, major vascular complications, and residual moderate or severe perivalvular leak were similar among the 3 groups.
Conclusions: After careful selection, transfemoral access is the preferential access route for TAVI procedures. In intermediate surgical risk patients with severe symptomatic aortic stenosis, non-transfemoral TAVI approaches have poorer outcomes. The worse outcomes of percutaneous alternative access routes are partially associated with worse baseline characteristics.
Keywords: Transcatheter aortic valve implantation. Transfemoral. Trans-subclavian. Transcaval.
RESUMEN
Introducción y objetivos: El acceso transfemoral (aTF) es el de elección para el implante percutáneo de válvula aórtica (TAVI). Sin embargo, hasta en el 15% de los pacientes este acceso es inadecuado. De las alternativas disponibles, el acceso totalmente percutáneo es el preferido. El objetivo del estudio es comparar los resultados clínicos de los pacientes tratados por aTF frente a los de aquellos con acceso transubclavio (aTS) o transcava (aTCv).
Métodos: Análisis retrospectivo de los pacientes tratados con TAVI (2008-2021) en un centro terciario mediante aTF, aTS y aTCv. El objetivo primario fue la mortalidad por cualquier causa a 30 días y 1 año. Los objetivos secundarios fueron el éxito técnico, la regurgitación perivalvular moderada o grave, las complicaciones vasculares mayores, el accidente vascular cerebral, el sangrado mayor y el daño renal agudo a 30 días (Valve Academic Research Consortium II [VARC-2]).
Resultados: Se realizaron 642 TAVI (601 por aTF, 24 por aTS y 10 por aTCv). Fueron excluidos 7 pacientes tratados por vía transapical. En los pacientes con acceso alternativo fue más frecuente la comorbilidad, incluyendo disfunción ventricular izquierda, enfermedad coronaria, fibrilación auricular, enfermedad renal e ictus previo. La mortalidad a 1 año y a 30 días fue también más alta en este grupo de pacientes (hazard ratio [HR] = 2,88 y 3,53, respectivamente). A 30 días, las tasas de ictus y daño renal (grado 2 o 3) fueron significativamente más bajas para el aTF, y similares en los pacientes con aTS y aTCv. Hubo también una tendencia a menor sangrado en el grupo de aTF. El éxito técnico, las complicaciones vasculares mayores y la regurgitación perivalvular moderada o grave fueron similares en los tres grupos.
Conclusiones: Tras una adecuada selección, el aTF es el preferido para el TAVI. En pacientes de riesgo intermedio con estenosis aórtica grave sintomática, un acceso no transfemoral tiene peores resultados. Los inferiores resultados del uso de vías percutáneas alternativas están parcialmente relacionados con las peores características clínicas basales.
Palabras clave: Implante percutáneo de válvula aórtica. Transfemoral. Transubclavio. Transcava.
Abbreviations
TAVI: implante percutáneo de válvula aórtica.
INTRODUCTION
Transfemoral access is globally accepted and advised by international guidelines as the preferred approach for transcatheter aortic valve implantation (TAVI).1-2 Despite the experience with this technique and miniaturization of the latest generation transcatheter heart valves (currently using 14-Fr-to-16-Fr sheaths), registries describe that transfemoral access simply cannot be used in about 15% to 20% of the patients, mainly due to heavily calcified peripheral arterial disease or unfavorable anatomy.3 Surgical access like the transapical and transaortic approaches are progressively being withdrawn due to their invasiveness and poor outcomes.4 To this date, no randomized clinical trials have been conducted comparing the different alternative percutaneous approaches (trans-subclavian, transcarotid, and transcaval) so their use depends on the center experience and the learning curve.5 Also, some approaches may be preferred in some patients depending on their comorbidities and vascular anatomy.
The trans-subclavian approach was first developed with a surgical exposure of the artery. However, more recently, a fully percutaneous trans-subclavian approach has been performed proving safe and feasible, making it potentially the second-line access following the transfemoral one. However, it may be contraindicated in the presence of vascular stenosis, tortuosity, increased angulation, and coronary artery bypass graft surgery using internal mammary artery grafts.6 The transcaval approach is the latest technique to be developed. It avoids the morbidity associated with the transthoracic surgical approach, and provides the same room ergonomics compared to transfemoral access, as well as the possibility of larger introducer sheaths via venous access without a higher risk of major bleeding.7 It requires a favorable abdominal anatomy and a very precise computed tomography scan to plan cavo-aortic puncture and aortic wall occlusion after delivery.8
Purpose
This analysis aims to compare the outcomes and complications of transfemoral, trans-subclavian, and transcaval access routes for TAVI.
METHODS
Patient selection
All patients referred for TAVI to a single tertiary center from 2008 through 2021 were included in this analysis. The transfemoral access was always considered the default access route. In patients in whom this access route was not possible, the alternative access route was decided by a heart team based on other clinical and anatomical features (calcified disease or extreme tortuosity of subclavian arteries, presence of a left internal mammary artery bypass graft, arteriovenous fistula in patients on hemodialysis, severe calcified abdominal aorta or other unfavorable conditions for cavo-aortic puncture. This analysis was approved by the center ethics committee and informed consent was obtained from all the patients.
Procedural protocol
Based on the center protocol, before TAVI, all patients underwent a transthoracic echocardiogram, a 12-lead electrocardiogram, lab tests, an invasive coronary angiography, and a preoperative computed tomography scan.
Regarding the trans-subclavian access, the left subclavian artery was often chosen due to its more favorable orientation unless the patient had a left internal mammary artery bypass graft. In the early cases, the artery used to be surgically exposed by a vascular surgeon but then the technique evolved to a fully percutaneous approach using Seldinger technique. In our own experience the subclavian artery hemostasis was achieved in all cases with suture-mediated closing devices (ProGlide).
Regarding the transcaval access, both a right femoral vein, and a left femoral artery (for secondary access) were used. Via femoral artery a pigtail catheter is initially advanced to perform an abdominal aorta angiography to later place it in the target entry site. Via femoral vein, using the telescope technique, a stiff 0.014 in coronary guidewire (Astato) is advanced inside a micro-guide catheter (FineCross) that is inside a 0.035 micro-guide catheter (NaviCross). Cavo-aortic puncture and crossing are performed with electrification of the guidewire and guidance of a snare placed in the abdominal aorta. The whole system is advanced from the inferior vena cava to the abdominal aorta allowing change to a 0.035 stiff guidewire (Lunquerquist). Afterwards, the sheath delivery system can be advanced over the wire, and the valve is implanted according to the standard technique. After valve implantation, the aortic wall defect is closed with an occlusion device (Amplatzer Duct Occluder). All patients were followed with regular visits 1, 3, and 12 months after TAVI, and thereafter yearly or with other periodicity based on assistant cardiologist decision.
Statistical analysis
Patients were categorized based on the access route used and analyzed based on the baseline characteristics, procedural data, and outcomes. Continuous variables were expressed as mean and standard deviation (SD) or median and interquartile ranges [IQR] when normal or non-normal distributions of data were found, respectively. Categorical variables were expressed as as absolute (n) and relative frequency (percentage). Normal distribution was confirmed using the Kolmogorov-Smirnov test or skewness and kurtosis. Inter-group differences were tested with an independent sample t-test for continuous variables of normal distribution, and the Mann-Whitney test for continuous variables without a normal distribution. Chi-square and Fisher’s exact test were used for categorical variables. The primary endpoints were evaluated using a Kaplan-Meier curve analysis and a Cox regression model for significant differences. Secondary outcomes were compared using Fisher’s exact test. Statistical significance was defined as P values < .05. All tests were two-sided. The software used for statistical analysis was SPSS version 25.0 (SPSS Inc., United States).
Endpoints definition
The primary endpoint was all-cause mortality at 30 days and 1 year between patients treated with transfemoral and non-transfemoral TAVI. Secondary outcomes included technical success, presence of residual moderate-to-severe paravalvular leak, major vascular complications, 30-day major bleeding, 30-day stroke assessed according to the VARC-2, and acute kidney injury (Acute Kidney Injury Network [AKIN] 2 or 3).
RESULTS
Population baseline characteristics
During the study period, 642 TAVIs were performed. A total of 7 patients were excluded from this analysis because their TAVI had been performed via transapical access. Of the remaining 635 ones, transfemoral, percutaneous non-transfemoral, trans-subclavian, and transcaval accesses were used in 601 (94.6%), 34 (5.4%), 24, and 10 patients, respectively. The baseline characteristics are shown on table 1. In the overall TAVI population, mean age was 82.0 ± 6.4 years old, and significantly lower in the non-transfemoral group. Women were much more prevalent in the transfemoral (57%) compared to the non-femoral group (30%). The severity of aortic stenosis was not significantly different among the subgroups of this analysis (mean gradient, 50mmHg; mean pulmonary artery systolic pressure, 44 mmHg; mean baseline New York Heart Association (NYHA) functional class, 2.76 ± 0.54; mean aortic valve calcium score by computed tomography of 2460 ± 1493 in the overall TAVI population). However, the prevalence of, at least, moderate left ventricular dysfunction was higher in the non-femoral population (21% of the patients with left ventricular ejection fraction < 40% vs 12% in the overall group). The prevalence of bicuspid aortic valve was not significant among the different groups. Of note, the prevalence of coronary artery disease tends to be higher in the non-femoral group (56% vs 41%, P = .093, respectively) with an expected lower rate of previous coronary artery bypass graft in the trans-subclavian group because the presence of an left internal mammary artery graft may preclude the use of the left subclavian access. In the 2 cases reported of trans-subclavian access in patients with previous coronary artery bypass graft, the right subclavian access was used. Higher prevalence of known atrial fibrillation, previous stroke, and chronic kidney disease was noted in the non-femoral group. EuroSCORE II and Society of Thoracic Surgery scores predicted statistically non-significant risks between both groups.
Table 1. Baseline characteristics
| Baseline characteristic | Overall TAVI population (635) | Transfemoral TAVI (601) | Non-transfemoral TAVI (34) | P |
|---|---|---|---|---|
| Age in years – mean ± SD | 82.0 ± 6.4 | 82.2 ± 6.3 | 78.9 ± 7.0 | .004 |
| Gender (female) | 57% (363) | 59% (354) | 27% (9) | < .001 |
| Coronary artery disease | 42% (265) | 41% (246) | 56% (19) | .093 |
| Previous MI | 16% (99) | 16% (93) | 18% (6) | .734 |
| Previous CABG | 14% (87) | 14% (82) | 15% (5) | .799* |
| Atrial fibrillation | 34% (214) | 33% (200) | 41% (14) | .333 |
| Previous stroke | 11% (70) | 10% (62) | 24% (8) | .019* |
| Diabetes | 35% (225) | 35% (213) | 35% (12) | .986 |
| CKD (KDIGO stage ≥ 3) | 49% (311) | 48% (290) | 62% (21) | .127 |
| Hemodialysis | 4% (17) | 4% (15) | 8% (2) | .306* |
| Previous pacemaker | 9% (58) | 9% (54) | 12% (4) | .540* |
| EuroSCORE II – mean [IQR] | 6.77 (5.27) | 6.72 (5.28) | 7.77 (7.55) | .125 |
| STS Score – mean [IQR] | 6.20 (3.87) | 6.25 (3.91) | 5.16 (3.30) | .579 |
| Basal NYHA class – mean ± SD | 2.76 ± 0.54 | 2.76 ± 0.54 | 2.79 ± 0.64 | .700 |
| Mean aortic gradient – mean ± SD | 50 ± 16 | 51 ± 16 | 47 ± 14 | .151 |
| PASP – mean ± SD | 44 ± 14 | 44 ± 14 | 46 ± 17 | .554 |
| LVEF < 50% | 22% (141) | 22% (129) | 35% (12) | .063 |
| LVEF < 40% | 12% (73) | 11% (66) | 21% (7) | .099* |
| Bicuspid aortic valve | 3% (21) | 3% (20) | 3% (1) | .909* |
| AVCS – mean ± SD | 2460 ± 1493 | 2448 ± 1488 | 2661 ± 1600 | .475 |
AVCS, aortic valve calcium score; CABG, coronary artery bypass graft; CKD, chronic kidney disease; KDIGO, kidney disease improving global outcomes; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; TAVI, transcatheter aortic valve implantation; PASP, pulmonary artery systolic pressure; SD, standard deviation; STS, Society of Thoracic Surgeons. | ||||
Primary endpoints
The 1-year and 30-day all-cause mortality rates were significantly higher in the non-transfemoral TAVI population with a hazard ratio of 3.53 (95% confidence interval [95%CI,1.48-8.43; P = .004) at 30 days, and 2.88 (95%CI, 1.56-5.28; P = .001) 1 year after TAVI. The highest mortality rate at 30 days after TAVI was seen in the transcaval population (30%) with no other deaths being reported within the first year after TAVI. In the trans-subclavian TAVI population, the mortality rate was high at 30 days (12.5%) and increased by a factor of 3 at 1 year (37.5%).
The 30-day and 1-year all-cause mortality Kaplan-Meier curves are shown on figure 1 and figure 2. Table 2 shows the mortality rates among the 3 groups of access routes for TAVI.
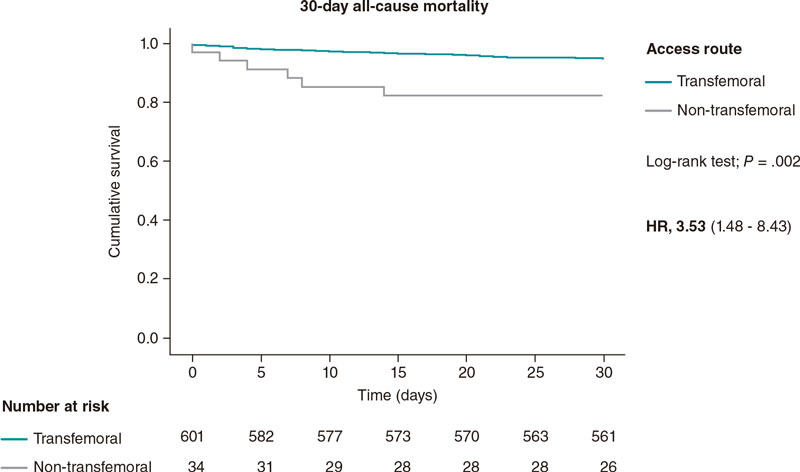
Figure 1. 30-day all-cause mortality Kaplan-Meier curve analysis between transfemoral and non-transfemoral TAVI patients. TAVI, transcatheter aortic valve implantation.
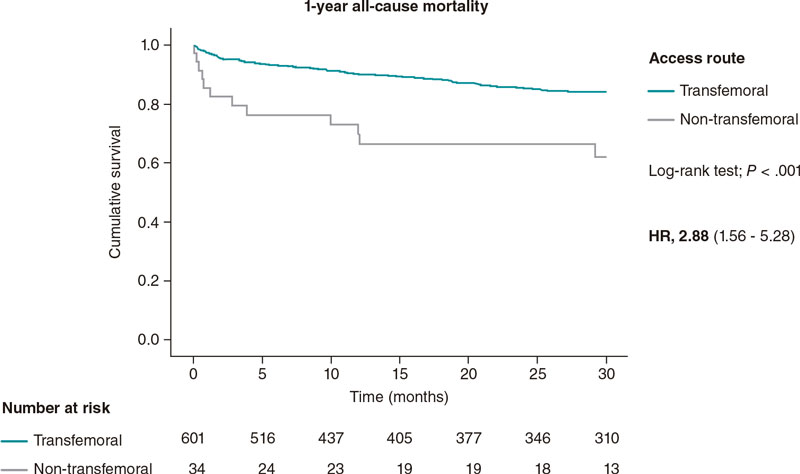
Figura 2 1-year all-cause mortality Kaplan-Meier curve analysis between patients treated with transfemoral and non-transfemoral TAVI. TAVI, transcatheter aortic valve implantation.
Table 2. Primary endpoints
| Primary endpoints | Transfemoral TAVI (601) | Non-transfemoral TAVI (34) | P | |
|---|---|---|---|---|
| TS (24) | TCv (10) | |||
| 30-day all-cause mortality | 5.4% (34) | 17.6% (6) | .015a | |
| 12.5% (3) | 30.0% (3) | |||
| 1-year all-cause mortality | 13.8% (82) | 35,3% (12) | .001b | |
| 37.5 % (9) | 30.0% (3) | |||
TAVI, transcatheter aortic valve implantation; TCv, transcaval; TS, trans-subclavian. | ||||
Secondary endpoints
The 30-day stroke and acute kidney injury (AKIN 2 or 3) rates were significantly lower in te transfemoral patients, but similar between trans-subclavian and transcaval patients. The 30-day rates of major bleeding showed a statistically significant tendency towards lower rates in the transfemoral group. The rates of technical success, major vascular complication, and residual moderate or severe paravalvular leak were similar among the 3 groups. Secondary endpoints are shown on table 3.
Table 3. Secondary endpoints
| Secondary endpoints | Transfemoral TAVI (601) | Non-transfemoral TAVI (34) | P | |
|---|---|---|---|---|
| TS (24) | TCv (10) | |||
| Technical success | 94% (562) | 94% (32) | .889* | |
| 92% (22) | 100% (10) | |||
| Residual moderate-to-severe leak | 3% (18) | 0% (0) | .616* | |
| 0% (0) | 0% (0) | |||
| Major vascular complication | 6% (34) | 9% (3) | .441* | |
| 8% (2) | 10% (1) | |||
| 30-day stroke | 3.5% (21) | 12% (4) | .039* | |
| 13% (3) | 10% (1) | |||
| 30-day major/life-threatening bleeding | 10% (60) | 21% (7) | .076* | |
| 17% (4) | 30% (3) | |||
| 30-day AKI (AKIN 2 or 3) | 8% (48) | 21% (7) | .022* | |
| 21 % (5) | 20% (2) | |||
AKIN, Acute Kidney Injury Network; TAVI, transcatheter aortic valve implantation; TCv, transcaval; TS, trans-subclavian. | ||||
DISCUSSION
In this retrospective analysis, we show data on the comparison between transfemoral and non-transfemoral access routes for TAVI in a tertiary center. The registered non-transfemoral rate of 5.4% was far below the rates described in the medical literature available (from 15% to 20%),3 which may be indicative of the recent developments and improvements made with TAVI sheaths and increasing technical experience gained leading to a lower need for alternative access routes in the current clinical practice. Nevertheless, this study shows that in high-volume centers, the number of patients with severe aortic stenosis who are ineligible for transfemoral TAVI remains a significant issue.
As expected, the transfemoral access route performed better than the alternative access routes regarding mortality rates, postoperative acute kidney injury and 30-day stroke and major bleeding events. No significant differences were seen regarding technical success, residual moderate-to-severe paravalvular leak or major vascular complications. This difference in outcomes can be explained, at least partially, by the dissimilar baseline characteristics. The primary and secondary endpoints were not significantly different between the trans-subclavian and the transcaval subgroups.
Without an interventional procedure being performed, severe aortic stenosis has poor prognosis, and a mortality rate somewhere around 50% at 2 years.9 Early studies of fully percutaneous trans-subclavian and transcaval TAVI reported 30-day mortality rates of 6% and 8%, respectively,6,7 higher than those reported for transfemoral TAVI. In addition, a systematic review of the medical literature available based on registries reported lower mortality rates for transfemoral TAVI vs other alternative access routes, at 30 days, with odds ratios (OR) of 0.56 and, at 1 year, and OR of 0.68 with similar rates of bleeding and cerebrovascular events.10 Aside from this differences in in-hospital and 30-day mortality, trans-subclavian approach is also associated with higher rates of acute myocardial infarction (OR, 5.3), renal complications (OR, 2.3), and pacemaker implantation (OR, 1.6) compared to transfemoral TAVI.11
A different registry reports similar 30-day and 1-year survival rates among transfemoral, transcaval, and transcarotid TAVI.12 These results were not found in our cohort probably due to the worse baseline characteristics and small number of transcaval procedures performed considering it’s a complex technique with a considerable learning curve.
In our analysis, the mortality registered 1 year after TAVI with non-transfemoral access is close to the mortality rates of severe aortic stenosis treated medically, which raises questions on the futility of these procedures. Furthermore, as seen on the baseline characterization of the population, the mean EuroSCORE II and Society of Thoracic Surgery scores (7.77% and 5.16%, respectively) stratifies the non-femoral population globally as intermediate-risk for surgical aortic valve replacement, a therapeutic option with 1-year mortality rates around 11% in patients with intermediate surgical risk aortic stenosis.13
Regarding the comparison between trans-subclavian and transcaval accesses, a retrospective analysis of a large multicentric registry describes lower rates of stroke (OR, 0.20; P = .014) and similar rates of bleeding (OR, 0.66; P = .38) with transcaval compared to trans-subclavian access.14 However, currently, there are no randomized data to support 1 specific alternative approach over the other. Hence, in patients with prohibitive transfemoral access route, vascular anatomy, risk factors, and the experience of the heart team should determine the preferred approach. Preoperative coronary computed tomography angiography plays a determinant role in the selection of the most appropriate alternative route as it allows us to accurately assess luminal diameters, vessels calcification, tortuosity and angulation, the previous use of 1 or 2 internal mammary arteries, and the aortic wall area eligible for cavo-aortic crossing.15
Limitations
This study has limitations associated with the nature of a non-randomized, retrospective, single center like selection bias. The extended period that was analyzed may also influence the results. In addition, the sample size in the transfemoral and transcaval subgroups is suboptimal, which limits the statistical analysis.
CONCLUSIONS
This analysis enhances the role of transfemoral access as the preferential access route for TAVI after careful patient selection. The trans-subclavian and transcaval approaches seem feasible with reasonable results. In intermediate surgical risk patients with severe symptomatic aortic stenosis, non-transfemoral TAVI has worse outcomes. The worse outcomes reported for percutaneous alternative access routes are partially associated with the worse baseline characteristics. In many of these patients, both the futility of the procedure and the experience of the heart team should be considered to determine the preferred approach. Further randomized clinical trials are needed to establish a preferential alternative access route in high surgical risk patients ineligible for transfemoral TAVI.
FUNDING
None whatsoever.
ETHICAL CONSIDERATIONS
The authors declare that this study was approved by the Institution and Institutional ethics committee. Informed consent was obtained from all patients for the analysis and publication of their data. Sex and gender variables have been taken into account in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence was not used for the development of this study or writing of the manuscript.
AUTHORS’ CONTRIBUTIONS
A. Grazina, B. Lacerda Teixeira, A. Castelo, T. Mendonça, and I. Rodrigues collected and analyzed data. A. Grazina, and B. Lacerda Teixeira drafted the manuscript with support from R. Ramos, A. Fiarresga, L. Patrício, D. Cacela, and R. Cruz Ferreira.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- Despite the experience gained with the technique and the miniaturization of the latest generation of transcatheter heart valves (currently using 14-Fr to 16-Fr sheaths), the transfemoral access is not feasible in about 15% to 20% of the patients.
- Regarding alternative access routes, fully percutaneous approaches (trans-subclavian/transaxillary, transcaval, and transcarotid) are preferred over surgical approaches (transaortic and transapical).
- To this date, no randomized clinical trials have ever been conducted comparing the different alternative percutaneous approaches.
- The use of alternative access routes depends on the center experience, the technique learning curve, and the patients’ comorbidities and vascular anatomy.
WHAT DOES THIS STUDY ADD?
- The transfemoral rate of 5.4% registered in this analysis is far lower compared to the one described in the medical literature, which may reflect the recent developments and improvements in TAVI sheaths and increasing technical experience gained leading to a lower need for alternative access routes in the current clinical practice.
- In our cohort, trans-subclavian and transcaval TAVI showed higher 30-day and 1-year mortality rates in intermediate risk patients compared to what has been reported in the medical literature for other options such as surgical aortic valve replacements.
- In high-risk or inoperable patients with severe aortic stenosis ineligible for transfemoral TAVI, trans-subclavian and transcaval approaches proved feasible. However, procedural futility should be considered in these cases.
- Trans-subclavian and transcaval approaches did not show any differences regarding mortality, technical success, residual moderate-to-severe leak, major vascular complications, or in the 30-day rates of stroke, acute kidney injury, and major bleeding.
REFERENCES
1. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–289.
2. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791.
3. Grover FL, Vemulapalli S, Carroll JD, et al. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69:1215–1230.
4. Overtchouk P, Modine T. Alternate Access for TAVI: Stay Clear of the Chest. Interv Cardiol. 2018;13:145-150.
5. Henn MC, Percival T, Zajarias A, et al. Learning Alternative Access Approaches for Transcatheter Aortic Valve Replacement: Implications for New Transcatheter Aortic Valve Replacement Centers. Ann Thorac Surg. 2017;103:1399–1405.
6. Schäfer U, Deuschl F, Schofer N, et al. Safety and efficacy of the percutaneous transaxillary access for transcatheter aortic valve implantation using various transcatheter heart valves in 100 consecutive patients. Int J Cardiol. 2017;232:247–254.
7. Greenbaum AB, O’Neill WW, Paone G et al. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol. 2014;63:2795–2804.
8. Greenbaum AB, Babaliaros VC, Chen MY et al. Transcaval Access and Closure for Transcatheter Aortic Valve Replacement: A Prospective Investigation. J Am Coll Cardiol. 2017;69:511–521
9. Kelly TA, Rothbart RM, Cooper CM, et al. Comparison of outcome of asymptomatic to symptomatic patients older than 20 years of age with valvular aortic stenosis. Am J Cardiol. 1988;61:123–130.
10. Chandrasekhar J, Hibbert B, Ruel M, et al. Transfemoral vs Non-transfemoral access for transcatheter aortic valve implantation: a systematic review and meta-analysis. Can J Cardiol. 2015;31:1427-1438.
11. Jiménez-Quevedo P, Nombela-Franco L, Muñoz-García E, et al. Early clinical outcomes after transaxillary versus transfemoral TAVI. Data from the Spanish TAVI registry. Rev Esp Cardiol. 2022;75:479-487.
12. Paone G, Eng M, Kabbani L, et al. Transcatheter Aortic Valve Replacement: Comparing Transfemoral, Transcarotid and Transcaval Access. Ann Thorac Surg. 2018;106:1105-1112.
13. Werner N, Zahn R, Beckmann A et al. Patients at Intermediate Surgical Risk Undergoing Isolated Interventional or Surgical Aortic Valve Implantation for Severe Symptomatic Aortic Valve Stenosis. Circulation. 2018;
138:2611-2623.
14. Lederman R, Babaliaros VC, Lisko JS, et al. Transcaval versus Transaxillary TAVR in Contemporary Practive: A Propensity-Weighted Analysis. JACC Cardiovasc Interv. 2022;15:965-975.
15. Biasco L, Ferrari E, Pedrazzini G, et al. Access sites for TAVI: Patient Selection, Criteria, Technical Aspects, and Outcomes. Front Cardiovasc Med. 2018;5:88.











