ABSTRACT
Introduction and objectives: Reperfusion therapy during an ST-segment elevation acute coronary syndrome (STEACS) can be performed using fibrinolytic agents or primary percutaneous coronary intervention (pPCI). The pPCI is the reperfusion strategy of choice, but many patients with STEACS initially come to non-PCI capable hospitals. Regional networks have been launched with both reperfusion therapies using thrombolysis in indicated cases followed by routine angiographic studies (pharmacoinvasive strategy). Our objective was to analyze the results of treatment in patients with STEACS in the Region of Murcia, Spain based on the patient’s place of origin.
Methods: Retrospective study of a cohort of patients admitted due to STEACS to 3 health areas: pPCI-capable Area 1 (Hospital Clínico Universitario Virgen de la Arrixaca), and non-pPCI capable Areas IV and V (Hospital Comarcal del Noroeste, Caravaca de la Cruz, and Virgen del Castillo, Yecla).
Results: Six hundred and seventy-nine patients from health areas I, IV, and V of the Region of Murcia were treated of STEACS from 2006 through 2010. Out of the 494 patients from Area I, 97.6% (482 patients) were treated with pPCI while 2.4% (12 cases) received thrombolysis. In Areas IV and V, 73% (135) of patients were treated with pPCI and 27% (50) with thrombolysis. After thrombolysis, 46 patients (34%) required rescue angioplasty and 79 (58.5%) underwent a scheduled coronary angiography (pharmacoinvasive strategy). No statistically significant differences were reported in the overall mortality rate at 30-day (8.3% in Area I vs 6% in Areas IV and V; P = .31) or 1 year follow-up (11.3% vs 8.2%; P = .23) in Area I compared to Areas IV and V, nor for cardiac mortality.
Conclusions: Although immediate pPCIs are less accessible in remote health areas, the healthcare network from the Region of Murcia can achieve similar mortality results compared to populations with pPCI availability.
Keywords: ST-segment elevation acute coronary syndrome. Reperfusion therapy. Fibrinolysis. Primary percutaneous coronary intervention.
RESUMEN
Introducción y objetivos: El tratamiento de reperfusión en un síndrome coronario agudo con elevación del segmento ST (SCACEST) se puede realizar con agentes fibrinolíticos o con angioplastia primaria (ICPp). La ICPp es la estrategia de elección, pero muchos de los pacientes con SCACEST acuden inicialmente a hospitales sin ICPp. Se han desarrollado programas de asistencia al SCACEST en los que se integran ambos tratamientos, utilizando la trombolisis en casos indicados, seguida de un estudio angiográfico (estrategia farmacoinvasiva). El objetivo del estudio es analizar los resultados del tratamiento del SCACEST según sea diagnosticado en áreas de salud con o sin disponibilidad de ICPp inmediata.
Métodos: Estudio retrospectivo de una cohorte de pacientes diagnosticados de SCACEST en 3 áreas de salud de Murcia: área I con ICPp (Hospital Clínico Universitario Virgen de la Arrixaca) y áreas IV y V sin ICPp (Hospital Comarcal del Noroeste, Caravaca de la Cruz y Virgen del Castillo, Yecla).
Resultados: Entre 2006 y 2010 se atendió por SCACEST a 679 pacientes de las áreas I, IV y V de Murcia. De los 494 pacientes del área I, recibieron tratamiento con ICPp el 97,6% (482) y trombolisis el 2,4% (12). En los pacientes de las áreas sanitarias IV y V se realizó trombolisis al 73% (135) e ICPp al resto 27% (50). De los pacientes sometidos a trombolisis, el 34% (46) precisaron angioplastia de rescate y al 58,5% (79) se les realizó coronariografía programada (estrategia farmacoinvasiva). No hubo diferencias en la mortalidad total a 30 días (8,3% en el área I y 6% en las áreas IV y V; p = 0,31) ni al año (11,3 frente a 8,2%; p = 0,23); tampoco en la mortalidad por causa cardiaca.
Conclusiones: A pesar de la menor accesibilidad a la ICPp en las áreas sanitarias más alejadas, la red asistencial regional de Murcia permite unos resultados comparables a los de las áreas sanitarias con disponibilidad de ICPp.
Palabras clave: Síndrome coronario agudo con elevación del segmento ST. Reperfusión. Fibrinolisis. Angioplastia primaria.
Abbreviations: pPCI: primary percutaneous coronary intervention. STEACS: ST-segment elevation acute coronary syndrome.
INTRODUCTION
The management of ST-segment elevation acute coronary syndrome (STEACS) is based on the quick opening of the culprit artery through the use of fibrinolytic drugs or a percutaneous coronary intervention (PCI) that limits the size of the infarction and improves prognosis.1 Fibrinolytic drugs have proven capable of increasing survival,2 but they are more effective when administered within the first 3 hours after symptom onset. The primary percutaneous coronary intervention (pPCI) improves survival and reduces recurrent infarctions and strokes, which is why it is seen as the optimal therapy as long as it can be performed in a timely manner.3,4
The pPCI main limitation is the impossibility to use it in the entire population due to its limited geographic availability and the delays involved in the transfer of patients from non-pPCI centers to reference hospitals. Clinical practice guidelines recommend performing pPCI < 120 min. after the diagnosis of STEACS.1 Regional networks have been created to speed up these times and increase access to pPCI for patients with STEACS in non-pPCI hospitals. Yet despite this effort, many patients with STEACS are transferred late to pPCI centers which increases mortality and morbidity rates.
In order to improve results and administer reperfusion therapy as early as possible the so-called pharmacoinvasive strategy was implemented. It consists of the administration of fibrinolytic drugs in the pre-hospital or non-pPCI setting followed by the immediate transfer of the patient to a pPCI center capable of performing a bailout angioplasty if drug therapy fails or an early systematic angiography if it is successful.5,6
The experience gained over the years performing pPCIs at the Hospital Clínico Universitario Virgen de la Arrixaca (HCUVA) has been used for the optimal management of patients with STEACS. The recommendations established by the clinical guidelines have been followed and adapted to the geographic characteristics of the region, structure, and healthcare resources available. A protocol for the management of reperfusion in the acute phase that distinguished 2 groups has been established: the first group with patients treated in pPCI centers; the second one, with patients from regional hospitals who live in remote areas far from reference hospitals where the treatment recommended is fibrinolysis in the absence of contraindications.
METHODS
Retrospective study of a cohort of 679 patients diagnosed with STEACS from 2006 through 2010 in 2 groups of healthcare regions: region I, with pPCI capabilities at the HCUVA (El Palmar, Murcia), and non-pPCI regions assigned to the HCUVA intensive care unit. This second group includes region IV with the Hospital Comarcal del Noroeste (Caravaca de la Cruz) and region V with the Hospital Virgen del Castillo (Yecla).
Patients diagnosed with STEACS based on traditional criteria1 and symptoms of less than 24-hour duration were included. Selection was done by reviewing the HCUVA catheterization laboratory database on all ICU admissions, hospital urgent care provided, and 061 ambulance emergency transfer reports during the study period. The most adequate reperfusion therapy was administered following recommendations and the regional protocol.
Follow-up was conducted by reviewing the patients’ medical records by phone or through physical consultations.
The variables analyzed were past medical history, time elapsed since symptom onset until reperfusion therapy, electrocardiogram, echocardiographic and angiographic characteristics of angioplasty, patient progression, and treatment after hospital discharge. Major hemorrhages were defined as lethal or symptomatic in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial or intramuscular) causing compartmental syndrome or bleeding with reduced hemoglobin levels > 20 g/L (1.24 mmol/L) or need for 2 concentrate transfusions.
The short and long-term cardiovascular events were recorded at the 30-day and 1-year follow-up, respectively including the rates of overall mortality and cardiac mortality, acute myo- cardial reinfarction (re-AMI), stroke, and need for a new revascularization.
The study primary endpoint was to compare mortality and major cardiovascular events in patients treated of STEACS from the Region of Murcia based on the healthcare region they received care at. The study secondary endpoints were the analysis and comparison of the clinical characteristics of these populations and the identification of angiographic or PCI differences.
Statistical analysis
The results of continuous variables were expressed as mean ± standard deviation, and those of categorical variables as frequency or percentage. Categorical variables were compared using the chi-square test with Yates correction when necessary. Quantitative variables were compared using the Student t test based on the variables normal distribution. Event-free survival rates (overall and cardiac mortality, stroke, re-AMI, and restenosis) were calculated using the Kaplan-Meier method and their results were represented through survival curves. The log rank test was used to compare the event-free survival rate. The level of statistical significance used for hypothesis testing was P < .05. The Mac OS version of the SPSS statistical software (version 20) was used.
The study was conducted in full compliance with the Declaration of Helsinki and the good clinical practice guidelines approved by HCUVA Research Ethics Committee.
RESULTS
From January 2006 through December 2010, 679 patients from regions I, IV, and V of the Region of Murcia Healthcare System were treated of STEACS of less than 24-hour duration and received reperfusion therapy (figure 1). Ninety-seven-point-six per cent of the 494 patients from region I (HCUVA) underwent pPCI (482) while 2.4% received thrombolysis (12). Seventy-three percent (135) and 27% (50) of patients from regions IV and V (127 and 58, respectively) underwent thrombolysis and pPCI, respectively. Thirty-four percent (46) of those who received thrombolysis required a bailout angioplasty and 58.5% (79) a scheduled coronary angiography (pharmacoinvasive strategy) during their hospital stay. Only 10 patients (7.4%) did not undergo a coronary angiography.
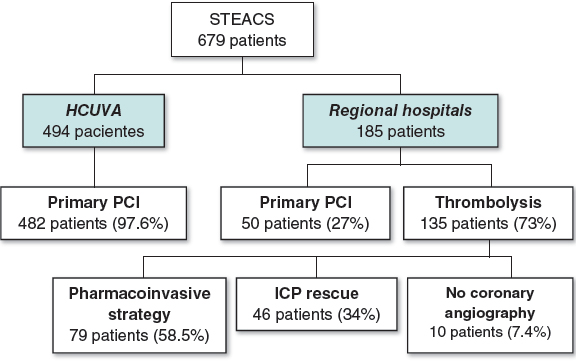
Figure 1. Summary of the study patients and the reperfusion strategies used based on the patients’ healthcare regions. HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca; PCI, percutaneous coronary intervention or angioplasty; STEACS, ST-segment elevation acute coronary syndrome.
Baseline characteristics of the populations
Baseline characteristics are shown on table 1. The HCUVA population was older and had more diabetic patients compared to the population from regional hospitals. On the contrary, the rate of atrial fibrillation was higher in the latter. No significant differences were seen based on sex or the remaining risk factors.
Table 1. Baseline characteristics of the population
| HCUVA (n = 494) | Regional hospitals (n = 185) | P | |
|---|---|---|---|
| Age (years) | 65.3 ± 13.7 | 62.9 ± 13 | .044 |
| Sex (women) | 111 (22.5) | 41 (22.2) | .93 |
| High blood pressure | 290 (58.7) | 111 (60) | .76 |
| Diabetes | 180 (36.4) | 52 (28.1) | .042 |
| Dyslipidemia | 200 (40.5) | 64 (34.6) | .16 |
| Smoking | 304 (61.5) | 112 (60.5) | .81 |
| Previous ischemic heart disease | 53 (10.7) | 21 (11.4) | .810 |
| Previous revascularization | 53 (10.7) | 18 (9.7) | .88 |
| Peripheral arterial disease | 25 (5.1) | 4 (2.2) | .096 |
| Previous stroke | 43 (8.7) | 12 (6.5) | .35 |
| Atrial fibrillation | 21 (4.3) | 16 (8.6) | .025 |
| Heart failure | 12 (2.4) | 2 (1.1) | .271 |
| Kidney disease | 39 (7.9) | 19 (10.3) | .324 |
| COPD | 43 (8.7) | 18 (9.7) | .677 |
| Valve disease | 9 (1.8) | 1 (0.5) | .217 |
| Previous angina | 122 (24.7) | 39 (21.1) | .324 |
COPD, chronic obstructive pulmonary disease; HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca. Data are expressed as no. (%) or mean ± standard deviation. | |||
No differences were seen in the time to reperfusion between both groups with a mean of 180 min. (interquartile range: [120-240]) in HCUVA vs 150 min. in regional hospitals (interquartile range: [90-240]; P = .4). Ischemia times < 3 hours were achieved in 59.6% of the HCUVA patients compared to 68.9% of patients from regional hospitals (table 2). Forty-nine patients (9.9%) from the first group had cardiogenic shock vs 17 patients (9.2%) from the second one (not statistically significant differences).
Table 2. Progression time (from symptom onset to reperfusion) and angiographic and electrocardiographic characteristics
| HCUVA (n = 494) | Regional hospitals (n = 185) | P | |
|---|---|---|---|
| Progression time(median,min.) | 180 | 150 | .4 |
| < 3 h | 295 (59.7) | 128 (69.1) | |
| 3-6 h | 141 (28.5) | 33 (17.7) | |
| 6-9 h | 32 (6.4) | 10 (5.6) | |
| 9h -12 h | 15 (3.1) | 7 (4) | |
| > 12 h | 11 (2.2) | 7 (4) | |
| STEACS location | .298 | ||
| Anterior | 205 (41.6) | 89 (48.1) | |
| Inferior | 236 (47.7) | 75 (40.5) | |
| Lateral | 49 (9.9) | 18 (9.7) | |
| Indeterminate | 4 (0.8) | 3 (1.6) | |
| Culprit vessel | .022 | ||
| Left anterior descending coronary artery | 205 (41.5) | 83 (44.9) | .429 |
| Circumflex artery | 62 (12.6) | 25 (13.5) | .738 |
| Right coronary artery | 204 (41.3) | 64 (34.6) | .111 |
| Left main coronary artery/graft | 9 (1.8) | 0 | .065 |
| Unidentified | 14 (2.8) | 13 (7) | .013 |
| Previous stent thrombosis | 24 (4.8) | 3 (1.6) | .075 |
| Number of injured vessels | .001 | ||
| 0 | 5 (1) | 15 (8) | .001 |
| 1 | 274 (55.4) | 109 (58.9) | .416 |
| 2 | 133 (27) | 38 (20.6) | .093 |
| 3 | 82 (16.6) | 23 (12.6) | .227 |
| Initial TIMI flow | .001 | ||
| 0 | 351 (71.1) | 65 (34.9) | .001 |
| 1 | 21 (4.2) | 4 (2.4) | .281 |
| 2 | 13 (2.7) | 9 (4.7) | .206 |
| 3 | 109 (22) | 107 (58) | .001 |
| Final TIMI flow grade 3 | 464 (93.9) | 171 (92.3) | .845 |
| Second revascularization | 95 (19.2) | 30 (16.2) | .322 |
| Complete revascularization | 347 (70.2) | 138 (74.6) | |
HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca; STEACS, ST-segment elevation acute coronary syndrome; TIMI, Thrombolysis in Myocardial Infarction. Data are expressed as no. (%) | |||
Regarding the coronary angiography, the percentage of radial access was similar: 45% and 48%, respectively. No significant differences were found either in the location of the STEACS (table 2). However, significant differences were seen in the culprit artery since it was a common thing to not be able to identify the vessel in patients from regional hospitals because the coronary arteries were patent. Differences were seen too in the initial TIMI flow (Thrombolysis in Myocardial Infarction) between both groups (P = .001) at the expense of a worse initial flow in HCUVA patients. After reperfusion therapy, TIMI flow grade-3 was achieved in the culprit artery in 93.9% of HCUVA patients and 92.3% of patients from regional hospitals. Revascularization was complete in 70.2% of the patients from region I and 74.6% of the patients from regions IV and V.
Analytic and echocardiographic characteristics and clinical progression
No differences were seen in the highest levels of cardiac necrosis markers between the different regions (table 3). On average the left ventricular ejection fraction was 52.15% in HCUVA patients and 52.29% in patients from regional hospitals without any significant differences in the systolic or diastolic function (table 3).
Table 3. Analytic, echocardiographic and disease progression characteristics at the hospital floor
| HCUVA (n = 494) | Regional hospitals (n = 185) | P | |
|---|---|---|---|
| Peak creatine kinase levels (µg/dL) | 1864.4 ± 1917.3 | 1938.3 ± 1834.4 | .671 |
| Peak creatine kinase-MB levels | 175.39 ± 132.34 | 182.26 ± 159.86 | .668 |
| Peak troponin T levels | 5.79 ± 9.4 | 9.38 ± 27.5 | .118 |
| Ejection fraction (%) | 52.15 ± 10.93 | 52.29 ± 11.46 | .886 |
| Normal | 255 (50.6) | 95 (51.5) | |
| Mild dysfunction | 152 (30.7) | 52 (28.1) | |
| Moderate dysfunction | 63 (12.8) | 32 (17.3) | |
| Severe dysfunction | 29 (5.9) | 6 (3.5) | |
| Diastolic pattern | .056 | ||
| Restrictive pattern | 19 (3.9) | 10 (5.3) | |
| Pseudo-normal pattern | 125 (25.3) | 33 (18) | |
| Prolonged relaxation | 307 (62.2) | 113 (61.3) | |
| Normal | 37 (7.6) | 23 (12.4) | |
| Atrial fibrillation | 5 (1.1) | 6 (3.3) | |
| Hospital stay (days) | 9.04 ± 5.72 | 9.81 ± 7.94 | .259 |
| Major hemorrhage | 11 (2.2) | 7 (3.8) | .261 |
| STEACS related complications | 6 (1.2) | 3 (1.6) | .71 |
| Killip Class I | 357 (72.3) | 154 (83.3) | .012 |
HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca; STEACS, ST-segment elevation acute coronary syndrome; Data are expressed as no. (%) or median ± standard deviation. | |||
No differences were seen in the rates of major bleeding and complications (cardiac ruptures: 2 and 2; intraventricular communication: 1 in regional hospitals, 2 in the HCUVA; papillary muscle rupture: 1 and 1). Patients from region I had more heart failure during their hospital stay (28.7% in the HCUVA vs 16.7% in regional hospitals).
30-day and 1-year follow-up results
Mean follow-up was 962 days in HCUVA patients and 1062 days in patients from regional hospitals. No differences were seen in the overall mortality or cardiac mortality rates at the 30-day or 1-year follow-up. No differences were seen either in the rates of AMI, stroke, and revascularization at the follow-up (table 4). Kaplan-Meier survival curves (figure 2) did not show any significant differences regarding mortality, cardiac death, AMI, and stroke.
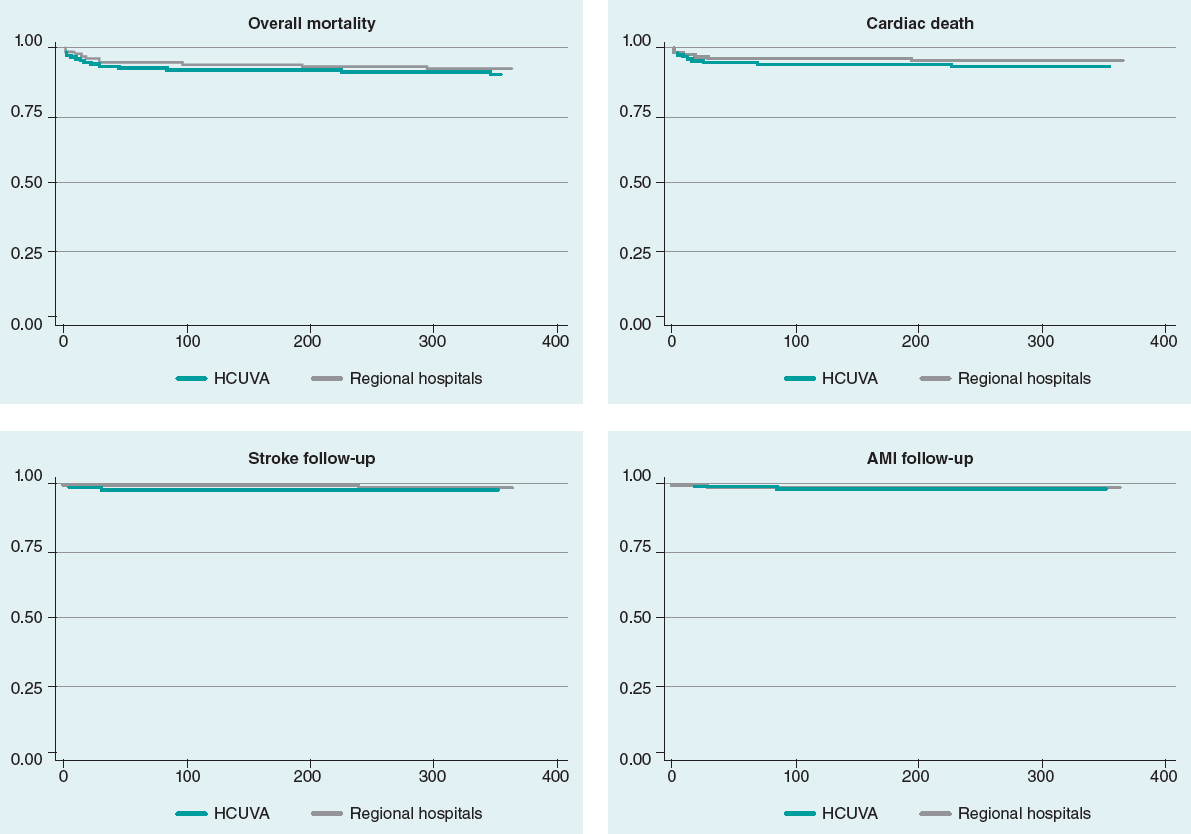
Figure 2. Survival curves. Mortality, cardiac death, stroke, and AMI at the follow-up. AMI, acute myocardial infarction; HCUVA, Hospital Clínico Universitario Virgen de la Arrixaca.
Table 4. Mortality and major cardiovascular events
| Results (%) | HCUVA (n = 494) | Regional hospitals (n = 185) | P |
|---|---|---|---|
| Mortality | |||
| 30 days | 41 (8.3) | 11 (6) | .312 |
| 1 year | 56 (11.3) | 15 (8,2) | .229 |
| Cardiac death | |||
| 30 days | 35 (7.1) | 8 (4.3) | .19 |
| 1 year | 43 (8.7) | 9 (4.9) | .095 |
| Reinfarction | |||
| 30 days | 7 (1.4) | 2 (1.1) | .735 |
| 1 year | 20 (4) | 5 (2.7) | .409 |
| Stroke | |||
| 30 days | 8 (1.6) | 3 (1.6) | .996 |
| 1 year | 15 (3) | 3 (1.6) | .309 |
| Revascularization | |||
| 30 days | 7 (1.4) | 4 (2.2) | .494 |
| 1 year | 35 (7.1) | 9 (4.9) | .294 |
HCUVA: Hospital Clínico Universitario Virgen de la Arrixaca. Data are expressed as no. (%). | |||
DISCUSSION
This study assessed the results of the management of STEACS from a population perspective and analyzed the consequences of the different care provided in each patient’s healthcare region. This was an observational and retrospective study conducted in 3 population areas from the Region of Murcia that share the same interventional cardiology unit and the same intensive care unit. A 5-year period was analyzed with an mean annual rate of 140 patients with STEACS who were admitted to the ER with symptoms of < 24-hour duration. To make the analysis more consistent and thorough, the past medical histories of patients admitted to their respective hospitals and the out-of-hospital ER system and 061 emergency service reports were reviewed to detect prehospital deaths.
The regional plan for the management of STEACS7 is part of the recommendation of designing regional networks beyond the idea of isolated hospital healthcare towards more comprehensive community healthcare systems including scientific recommendations, geographical peculiarities, resources and infrastructures available, and the characteristics of healthcare organization. This plan suggests initiating reperfusion therapy as early as possible whether mechanical with pPCI o pharmacological with fibrinolysis.
The pPCI is considered the treatment of choice for patients admitted to the ER within 60 min. since symptom onset.1,8,9 This is how patients diagnosed with STEACS in the metropolitan area of Murcia and nearby municipalities are treated.7 For remote areas such as healthcare regions IV and V, fibrinolytic therapy is recommended in the absence of contraindications followed by transfer to the HCUVA ICU plus urgent coronary angiography in the absence of reperfusion signs (bailout PCI) or elective coronary angiography within the first 24 hours to 48 hours (pharmacoinvasive strategy).7 The hospitals from such areas are 75 km and 110 km away (figure 3) respectively from the pPCI reference hospital.
Figure 3. Healthcare regions within the Region of Murcia, Spain.
Populations from pPCI-capable regions (494 patients) and those from remote regions (185 patients) are rather similar: 78% males, many diabetic patients (> 28%), and over 60% smokers. The only differences between both groups are that patients from region I are older and have a higher prevalence of diabetes (36.4% vs 28.1%). The percentage of diabetics in this series is higher compared to that of international studies like the STREAM trial (12.1% to 13.1%) and other national studies like those conducted by Rodríguez-Leor et al.10 (24.8%), and Hernández-Pérez et al.11 (19.1%), and similar to the EUROASPIRE-IV registry (27%).12
The studies conducted until 2006 in patients with STEACS admitted to the ER in a timely manner showed that up to 25% to 30% did not receive reperfusion therapy.13,14 This has improved with the implementation of STEACS care networks. Proof of this are the results from several networks in Europe and the United States with percentages from 100% (the Mayo Clinic network)15 to 84% (the Alberta network, Canada).16 Our data are indicative of a high percentage of reperfusion therapy in the studied regions.
In region I the pPCI was performed in almost all of the cases (97.6%) while in the remaining 2 regions 27% of the 185 patients were referred to other centers for mechanical reperfusion. The existence of contraindications for thrombolysis, the long progression time or the possibility of agile hospital transfers to the interventional cardiology unit facilitated the performance of pPCI in 1 out of every 4 patients with STEACS from these regions; the rest (73%) received fibrinolysis. These data are indicative of a greater use of fibrinolytic therapy compared to the one reported by other studies. Thus, a Belgium registry17 reported that fibrinolytic therapy was prescribed to 28.7% of the population from regional hospitals over the first few years (2007-2008). However, this percentage dropped to 12.6% over the last few years (2009-2010). The higher percentage of thrombolytic therapy seen in our study is associated with a longer distance between regional hospitals and the reference pPCI hospital. Even so, over the last few years, a higher percentage of patients with STEACS referred to pPCI centers has been reported in our region. At the program early stages,18 in healthcare regions IV and V, the percentage of pPCIs performed was between 1% and 2% of all reperfusion therapies. In our study, this percentage grew to 27% after reducing patient transfer times between hospitals.
Coronary angiography was performed in 95% of the patients who received fibrinolytic therapy, a similar percentage compared to that reported by other registries (96% in the FAST-MI,19 and 97% in the Mayo Clinic Care Network registry15) and higher to the one reported by the Belgium registry (69%).17
Reperfusion mean times are also similar to those reported by the registries mentioned above. Time delay until reperfusion therapy was < 3 hours in 59.6% of the patients from region I and 68.9% of the patients from regions IV and V. These are similar rates to those from the Belgium trial17 in which the time elapsed since symptom onset until reperfusion therapy was < 4 hours in 67% of the patients from pPCI hospitals and 63% of the patients from regional hospitals and to those from the Mayo Clinic Care Network AMI protocol.15 This protocol establishes a pharmacoinvasive strategy where total ischemia times were 103 min. in patients who received thrombolysis and 278 min. in those referred to undergo pPCI (with a mean time until reperfusion in regional hospitals of 181 min.).
No differences were seen in the location of the infarction between both groups. Patients referred from regional hospitals had more coronary arteries without lesions and a higher preprocedural rate of TIMI flow grade-3 compared to a higher rate of occluded infarct related culprit arteries in those referred for pPCI. Upon arrival to the catheterization laboratory, the initial TIMI flow grade was 0-1 in 75.6% of the patients referred for pPCI and 37.3% in those who received thrombolysis. Different studies show that when the coronary angiography is performed there is a higher percentage of patients with TIMI flow grade-3 among patients who received thrombolysis.20
Clinical progression was similar with no differences regarding major bleeding complications (2.2% vs 3.8%), stroke (1.6% vs 1.6% at 30 days), re-AMI (1.4% vs 1.1% at 30 days), and need for revascularization (1.4% vs 2.2% at 30 days, 7.1% vs 4.9% at 1 year). However, the rate of heart failure during the hospital stay was higher in HCUVA patients (27.3% vs 16.7%). This result may be explained by a tendency towards a greater grade of advanced diastolic dysfunction in these patients (25.3% vs 18%). However, despite the longer ischemia time there were no significant differences in the AMI size due to systolic dysfunction or peak creatinine kinase-MB levels with peak values of 175 vs 182 µg/dL.
The mortality of patients looked after in regions assigned to non-pPCI regional hospitals is similar to that of patients looked after in the reference pPCI hospital. At 1-month, the overall mortality rate was 8.3% in region I with pPCI capabilities and 6% in the most remote areas assigned to regional hospitals; cardiovascular mortality rate was 7.1% and 4.3%, respectively. These rates are similar to those reported by other studies conducted in our setting like the 7.5% from the RESCATE II,21 7.26% from the RECALCAR trial,22 11% from the PRIAMHO-II trial,23 and 7.6% from the MASCARA trial.24 They are also similar to those from the Belgium infarction care network17 where the mortality rates of regional and pPCI hospitals were 7% and 6.7%, respectively or the Mayo Clinic AMI Care Network where the mortality rates of patients from regional hospitals and pPCI hospitals were 5.2% and 7.2%, respectively.15
Based on these findings a reflection is to be made on some of the things that worry healthcare providers, Administration, and patients such as accessibility and equity in the healthcare system. In the STEACS setting there is an ongoing debate on how to make pPCI available for the entire population. Data from this and other studies,19,25 show that even if pPCI is the preferred reperfusion strategy, it is not the only one. In patients looked after in remote areas far from hospitals with experienced heart teams a pharmacoinvasive strategy with fibrinolytic treatment in the absence of complications is a good alternative.
Limitations
The scarce population from regions IV and V brings down the annual number of patients with STEACS, which is why the timeframe studied had to be a large one in order to study a representative sample. This was a retrospective analysis with the limitations of this type of studies. Basically, this shows how difficult it was to obtain certain data like those regarding different timeframes. The findings from this study where patients were always transferred to the reference hospital intensive care unit may vary from those of other regions where delays could occur if fibrinolysis was not successful. Another possible limitation would be that only patients treated with reperfusion therapy were studied. As already discussed, patients who may have died during the transfer or at the ER were searched for to discard differences in the results obtained from patients assigned to a reperfusion strategy and those finally treated. However, patients with STEACS who did not receive reperfusion therapy were not studied (cases with long symptom duration, etc.). The study compared the results based on the patients’ healthcare region, which may be decisive when assessing the management of STEACS in different healthcare regions, and the different ways of administering various types of reperfusion therapy. This does not seem to be a problem at the moment since reperfusion therapy is administered to over 80% of the cases without significant regional differences.
CONCLUSIONS
Patients diagnosed with STEACS from the most remote healthcare regions of the Region of Murcia (regions IV and V) show similar clinical characteristics compared to patients from region I. However, they are younger patients with not so much diabetes. Yet despite the lower accessibility to immediate pPCI for populations from these healthcare regions, the regional network gives results that are similar to those of populations from pPCI-capable healthcare regions. Pharmacoinvasive strategy is a valid reperfusion therapy for populations from non-pPCI healthcare regions within the times recommended, with similar survival rates to those of pPCI regions, without a higher rate of complications, and with similar short and long-term results.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Fibrinolysis and pPCI are reperfusion therapies for the management of STEACS. The latter is superior to the former if performed in a timely manner and under the right conditions.
- The pPCI main limitation is that it is impossible to offer it to the entire population due to time delays and availability issues.
- Regional networks have been created to reduce time to reperfusion and increase the availability of pPCI.
- Yet despite this effort, some patients with STEACS do not make it on time to the ER to be treated with pPCI. This delay is associated with higher mortality and morbidity rates.
WHAT DOES THIS STUDY ADD?
- Accessibility to pPCI for patients diagnosed with STEACS from remote areas is much lower.
- Being part of a healthcare regional network gives results that are similar to those of populations from pPCI-capable regions.
- This study shows that in an infarction care regional network system, reperfusion therapy can be performed by combining pharmacoinvasive strategy and pPCI.
- That is the way to achieve survival rates similar to those of patients who live close to pPCI-capable hospitals without a higher rate of complications.
REFERENCES
1. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
2. Fibrinolytic Therapy Trialists'(FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction:collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet. 1994;343:311-322.
3. Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733-742.
4. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction:a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20.
5. Borgia F, Goodman SG, Halvorsen S, et al. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST-segment elevation myocardial infarction:a meta-analysis. Eur Heart J. 2010;31:2156-2169.
6. Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379-1387.
7. Servicio Murciano de Salud, Consejería de Sanidad y Consumo. Programa Integral de Atención a La Cardiopatía Isquémica 2010-2013. 2010. Disponible en:https://www.murciasalud.es/publicaciones.php?op=mostrar_publicacion&id=1771&idsec=88. Consultado 20 Dic 2020.
8. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-140.
9. Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization:The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2014;35:2541-2619.
10. Rodriguez-Leor O, Fernandez-Nofrerias E, Mauri F, et al. Analysis of reperfusion delay in patients with acute myocardial infarction treated with primary angioplasty based on first medical contact and time of presentation. Rev Esp Cardiol. 2011;64:476-483.
11. Hernandez-Perez FJ, Blasco-Lobo A, Goicolea L, et al. Use of the radial approach in primary angioplasty:results in 1029 consecutive patients and analyses in unfavorable subgroups. Rev Esp Cardiol. 2014;67:45-51.
12. Kotseva K, Wood D, De Bacquer D, et al. EUROASPIRE IV:a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol. 2016;23:636-648.
13. Eagle KA, Nallamothu BK, Mehta RH, et al. Trends in acute reperfusion therapy for ST-segment elevation myocardial infarction from 1999 to 2006we are getting better but we have got a long way to go. Eur Heart J. 2008;29:609-617.
14. Gibson CM, Pride YB, Frederick PD, et al. Trends in reperfusion strategies, door-to-needle and door-to-balloon times, and in-hospital mortality among patients with ST-segment elevation myocardial infarction enrolled in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1035-1044.
15. Ting HH, Rihal CS, Gersh BJ, et al. Regional systems of care to optimize timeliness of reperfusion therapy for ST-elevation myocardial infarction:the Mayo Clinic STEMI Protocol. Circulation. 2007;116:729-736.
16. Shavadia J, Ibrahim Q, Sookram S, Brass N, Knapp D, Welsh RC. Bridging the gap for nonmetropolitan STEMI patients through implementation of a pharmacoinvasive reperfusion strategy. Can J Cardiol. 2013;29:951-959.
17. Claeys MJ, Sinnaeve PR, Convens C, et al. STEMI mortality in community hospitals versus PCI-capable hospitals:results from a nationwide STEMI network programme. Eur Heart J Acute Cardiovasc Care. 2012;1:40-47.
18. Valdés Chávarri M, Pinar Bermúdez E, Lacunza Ruiz J, et al. The primary percutaneous coronary intervention program in Murcia. Rev Esp Cardiol Supl. 2011;11(C):28-34.
19. Danchin N, Coste P, Ferrieres J, et al. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction:data from the french registry on acute ST-elevation myocardial inf. Circulation. 2008;118:268-276.
20. Giannopoulos G, Pappas L, Synetos A, et al. Association of virtual histology characteristics of the culprit plaque with post-fibrinolysis flow restoration in ST-elevation myocardial infarction. Int J Cardiol. 2014;174:678-682.
21. Garcia-Garcia C, Sanz G, Valle V, et al. Trends in in-hospital mortality and six-month outcomes in patients with a first acute myocardial infarction. Change over the last decade. Rev Esp Cardiol. 2010;63:1136-1144.
22. Bertomeu V, Cequier A, Bernal JL, et al. In-hospital mortality due to acute myocardial infarction. Relevance of type of hospital and care provided. RECALCAR study. Rev Esp Cardiol. 2013;66:935-942.
23. Aros F, Loma-Osorio A, Vila J, et al. Effect of combined beta-blocker and angiotensin-converting enzyme inhibitor treatment on 1-year survival after acute myocardial infarction:findings of the PRIAMHO-II registry. Rev Esp Cardiol. 2006;59:313-320.
24. Ferreira-Gonzalez I, Permanyer-Miralda G, Marrugat J, et al. MASCARA (Manejo del Sindrome Coronario Agudo. Registro Actualizado) study. General findings. Rev Esp Cardiol. 2008;61:803-816.
25. Larson DM, Duval S, Sharkey SW, et al. Safety and efficacy of a pharmaco-invasive reperfusion strategy in rural ST-elevation myocardial infarction patients with expected delays due to long-distance transfers. Eur Heart J. 2012;33:1232-1240.
Corresponding author: Hospital Clínico Universitario Virgen de la Arrixaca, Sección de Hemodinámica, Servicio de Cardiología, Carretera Cartagena-El Palmar, 30120 El Palmar, Murcia, Spain.
E-mail address: epbhva@yahoo.es (E. Pinar Bermúdez).











