ABSTRACT
Introduction and objectives: Coronary calcification is one of the leading factors that affect negatively the safety and effectiveness of percutaneous coronary intervention. Several calcium modification techniques exist. However, there is a lack of randomized evidence on the therapy of choice in this scenario.
Methods: The ROLLERCOASTR is a prospective, multicenter, randomized clinical trial designed to compare the safety and efficacy profile of 3 plaque modification techniques in the moderate-to-severe coronary calcification setting: rotational atherectomy (RA), excimer laser coronary angioplasty (ELCA), and intravascular lithotripsy (IVL). The study primary endpoint is stent expansion evaluated by optical coherence tomography. An intention-to-treat analysis will be conducted with an alpha coefficient of 0.05 between the reference group (RA) and the remaining 2 groups (ELCA and IVL). An analysis of the study primary endpoint per protocol will be conducted for consistency purposes. If the non-inferiority hypothesis is confirmed, a superiority 2-sided analysis will be conducted. Both the clinical events committee and the independent core laboratory will be blinded to the treatment arm. Assuming an α error of 0.05, an β error of 0.2 (80% power), a margin of irrelevance (ε) of 7, and losses of 10% due to measurement difficulty or impossibility to complete the intervention, we estimate a sample size of 56 cases per group. The study secondary endpoints are device success, procedural success, crossover rate among the different techniques used, and the occurrence of major adverse cardiovascular events at 1-year follow-up.
Conclusions: The ROLLERCOASTR trial will evaluate and compare the safety and effectiveness of 3 plaque modification techniques: RA, ELCA, and IVL in patients with calcified coronary stenosis. This trial was registered at clinicaltrials.gov with identifier NCT04181268.
Keywords: Percutaneous coronary intervention. Calcified plaques. Laser. Lithotripsy. Rotational atherectomy. Optical coherence tomography.
RESUMEN
Introducción y objetivos: La calcificación coronaria es uno de los principales factores que inciden negativamente en la seguridad y la eficacia del intervencionismo coronario percutáneo. Existen varias técnicas de modificación del calcio, pero falta evidencia de estudios aleatorizados sobre la terapia de elección en este escenario.
Métodos: El ROLLERCOASTR es un estudio prospectivo, multicéntrico y aleatorizado, diseñado para comparar la seguridad y la eficacia de 3 técnicas de modificación de la placa en el contexto de calcificación coronaria moderada o grave: aterectomía rotacional (AR), aterectomía coronaria con láser láser excimer (ACLE) y litotricia intracoronaria (LIC). El objetivo primario es la expansión del stent evaluada mediante tomografía de coherencia óptica. Su análisis se hará por intención de tratar, con un α de 0,05 entre el grupo de referencia (AR) y cada uno de los otros grupos (ACLE y LIC). Se realizará también un análisis del objetivo primario por protocolo para mantener la coherencia. Si se confirma la hipótesis de no inferioridad, se realizará un análisis bilateral de superioridad. El comité de eventos clínicos y el laboratorio central independiente no conocerán la rama de tratamiento. Asumiendo un error α de 0,05, un error β de 0,2 (80% de potencia), un margen de irrelevancia (ε) del 7% y un 10% de pérdidas por dificultad de medición o imposibilidad de completar la intervención, se estima un tamaño de muestra de 56 casos en cada grupo. Los objetivos secundarios son el éxito del dispositivo, el éxito del procedimiento, la tasa de cruce entre técnicas y la presentación de eventos cardiovasculares adversos importantes al año de seguimiento.
Conclusiones: El estudio ROLLERCOASTR evaluará y comparará la seguridad y la eficacia, en pacientes con estenosis coronaria calcificada, de 3 técnicas de modificación de placa: AR, ACLE y LIC. Este ensayo se ha registrado en Clinicaltrials.gov: NCT04181268.
Palabras clave: Intervencionismo coronario percutáneo. Placas calcificadas. Láser. Litotricia. Aterectomía rotacional. Tomografía de coherencia óptica.
Abbreviations
DES: drug-eluting stent. ELCA: excimer laser coronary angioplasty. IVL: intravascular lithotripsy. OCT: optical coherence tomography. PCI: percutaneous coronary intervention. RA: rotational atherectomy.
INTRODUCTION
Percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation is the most frequent mode of coronary revascularization.
Calcified coronary lesions pose a challenge to perform successful PCI.1 Coronary calcification impedes PCI by multiple mechanisms like limiting DES lesion crossing, altering the drug elution kinetics, and interfering with optimal stent expansion. In addition, inadequate stent expansion is a powerful predictor of stent thrombosis and restenosis.2-6 Coronary calcification also increases PCI-related procedural complications (dissection, perforation, myocardial infarction), and late adverse clinical outcomes like restenosis, repeat revascularization, stent fracture, and thrombosis.1 The optimal approach for the management of calcified stenosis requires taking into account the characteristics of the lesion, calcium distribution, and the mechanism of action of every plaque-modification device. In this regard, intracoronary imaging techniques such as intravascular ultrasound and optical coherence tomography (OCT) are essential not only to evaluate the severity of calcification and its pattern, but also to optimize stenting.7
Currently, plaque-modification techniques can be categorized into a) balloon-based technologies (cutting/scoring balloons, non-compliant and super high-pressure balloons, and intravascular lithotripsy (IVL), and b) non-balloon-based technologies (rotational atherectomy [RA], orbital atherectomy, and excimer laser coronary angioplasty [ELCA]).8,9
The widespread use of these techniques and devices has been limited due to the risk of complications, the operator’s experience, and the corresponding use of health resources. Over the past few decades, RA has been the therapy of choice for resistant calcified lesions. However, the development of new technologies such as IVL or the improvement of classical therapies such as ELCA has generated uncertainty on the optimal tool to modify calcified plaques as non-randomized comparisons between these techniques have been drawn.
The objective of this randomized trial is to assess the efficacy and safety profile of intensive plaque modification with RA, IVL or ELCA before DES implantation.
METHODS
Patients and study design
The ROLLERCOASTR (Rotational atherectomy, lithotripsy or laser for the treatment of calcified stenosis) is an investigator-initiated, multicenter, prospective, and randomized clinical trial that includes 6 large volume sites. Also, it includes men and women aged ≥ 18 years with a clinical indication for PCI (stable or unstable ischemic heart disease) in vessels with reference diameters ≥ 2.5 and ≤ 4.0 mm and moderate-to-severe calcification estimated by coronary angiography. The main study exclusion criteria are ST-segment elevation acute coronary syndrome as clinical presentation, cardiogenic shock, inability to tolerate dual antiplatelet therapy for, at least, 6 months for those who are not on oral anticoagulation, impossibility to obtain informed consent from the patient or conduct, at least, a 1-year follow-up.
Patients who meet all the inclusion criteria and none of the exclusion ones will be randomized on a 1:1:1 ratio to either lesion preparation with RA, ELCA or IVL. Randomization will on a web-based platform. The complete inclusion and exclusion criteria are shown on table 1 while the study flowchart is described on figure 1.
Table 1. Study inclusion and exclusion criteria
| Inclusion criteria |
|---|
| ≥ 18 years old |
| Diameter stenosis ≥ 70% or fractional flow reserve < 0.8/non-hyperemic indexes < 0.89 |
| Reference vessel diameter ≥ 2.5 and ≤ 4 mm |
| Moderate or severe calcification estimated by coronary angiography |
| Patients with stable coronary artery disease or non-ST-segment elevation acute coronary syndrome |
| Culprit lesions at native vessels or coronary bypasses |
| Exclusion criteria |
| Inability to tolerate a 6-month course of dual antiplatelet therapy in patients naïve to oral anticoagulation |
| ST-segment elevation acute coronary syndrome |
| Cardiogenic shock |
| Impossibility to obtain informed consent from the patient or his legal representative |
| Impossibility to conduct, at least, a 1-year follow-up |
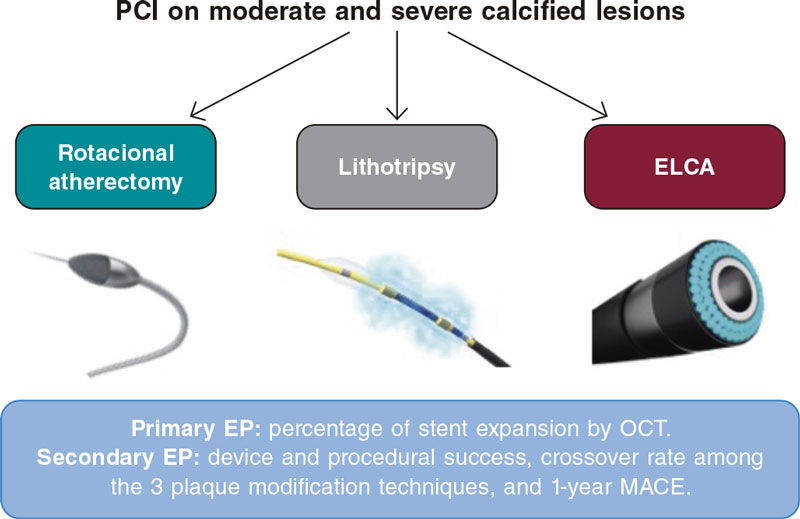
Figure 1. Study flowchart. ELCA, excimer laser coronary angioplasty; EP, endpoint; MACE, major adverse cardiovascular events; OCT, optimal coherence tomography; PCI, percutaneous coronary intervention.
Study primary and secondary endpoints
The objective of this study is to evaluate and compare the results of RA, IVL, and ELCA for the management of calcified coronary lesions. This comparison will be made by assessing the angiographic and OCT findings after the implementation of these plaque modification techniques, and DES implantation and optimization.
The primary endpoint is the comparison between RA (reference group) vs ELCA and RA vs IVL in the percentage of stent expansion measured using OCT. As secondary endpoints we’ll be analyzing the device success (successful stent implantation with minimum stent area ≥ 5.5 mm2, final TIMI grade-3 flow, and no need for another plaque preparation strategy), procedural success (device success and no severe procedural complications like cardiovascular death, perioperative target vessel myocardial infarction, need for new target lesion revascularization, stent thrombosis, stroke or vessel perforation with extravasation [types II or III]), crossover from the assigned plaque modification technique to a different one, and occurrence of major adverse cardiovascular events at 1-year follow-up (cardiovascular death, target vessel myocardial infarction, target lesion revascularization or stent thrombosis). We’ll also be analyzing device success regarding the type of calcified plaque (concentric, eccentric, calcium nodule). The study primary and secondary endpoints are shown on table 2.
Table 2. Study main endpoints
| Primary endpoint |
|---|
| Percentage of stent expansion measured by OCT |
| Key secondary endpoints |
| Device success (successful stent implantation with minimum stent area ≥ 5.5 mm2, final TIMI grade-3 flow, and no need for another plaque preparation strategy) |
| Device success depending on the type of the calcific plaque: concentric, eccentric or nodular |
| Procedural success (device success in the absence of procedural severe complications) |
| Crossover from the assigned plaque modification technique to a different one |
| 1 year-MACE (CD, TVMI, TLR or ST) |
CD, cardiac death; MACE, major adverse cardiovascular events; OCT, optical coherence tomography; ST, stent thrombosis; TLR, target lesion revascularization; TVMI, target vessel myocardial infarction. |
Devices
- – RA: Rotablator or RotaPro System (Boston Scientific, Unites States).
- – Coronary laser: Coronary laser-emitting device (CVX-300 ELCA System, Spectranetics Inc., United States).
- – Intracoronary lithotripsy: Shockwave System, (Shockwave Medical, United States).
- – OCT system: OCT Imaging system (Abbott Vascular, United States)
- – Stents: new-generation DES are mandatory (those currently being used in participant centers during the inclusion period).
Procedure
The angioplasty will be performed following the recommendations established by the current clinical practice guidelines on the management of coronary revascularization.10 After crossing the lesion with the angioplasty guidewire, a first OCT assessment should be performed. If necessary, balloon dilatation is allowed to cross the OCT catheter. After this first OCT pullback, the use of a plaque modification technique will be required (RA, laser or lithotripsy) on a randomized basis. Afterwards, a second OCT assessment is advised to analyze the effects of the therapy. Finally, the angioplasty will be completed with the implantation of a new-generation DES. Pre or postdilatation will be left to the operator’s criterion. After stenting (in the absence of postdilatation) or after the last postdilatation (if performed), a final OCT pullback will be performed to assess the final stent expansion.
Rotational atherectomy technique
The lesion will be crossed using the RotaWire (Boston Scientific, Unites States) directly or microcatheters or coaxial balloons. The RotaWire type (RotaWire Extra Support and RotaWire Floppy) will be used based on the characteristics of the plaque, the support required, and the operator’s preferences. Afterwards, the rotational atherectomy technique will be used based on the current recommendations.11 A 0.5:0.6 ratio between the burr and the vessel is advised. The rotational speed recommended is between 135 000 rpm and 180 000 rpm. Decelerations > 5000 rpm should be avoided. The burr should be advanced gradually with easy back-and-forth moves. Rotablation time should be < 20 seconds with pauses in between each cycle. Once rotablation has been performed, the burr should be removed with the Dynaglide mode on.
Intracoronary lithotripsy technique
The Shockwave balloon (Shockwave Medical, Inc., United States) is a 12 mm-long angioplasty balloon with 2.5 mm to 4 mm diameters. It can be mounted over a 0.014 in guidewire. Mechanical energy is transmitted to the lesion when the Shockwave balloon contacts the artery intima layer and cracks superficial and deep calcium layers. Therefore, the Shockwave balloon/reference vessel diameter ratio should be 1:1.12 Performing an OCT assessment prior to selecting the size of the balloon is also advised. Predilatation with balloons of smaller diameters is allowed to facilitate the passage of the lithotripsy balloon.
Once the Shockwave balloon is on the lesion, it is inflated at a pressure of 4 atm . Up to 80 pulses per balloon can be administrated (8 runs of 10 pulses). After every run (≤ 10 pulses), the Shockwave balloon is inflated at 6 atm and, after deflation, a new cycle can be applied if necessary. A minimum of 20 pulses per lesion is advised.
Laser technique
The size of the ELCA catheter will be selected considering the diameter of the target vessel on a 0.5-0.6 ratio with respect to its diameter.13 However, 0.9 mm catheters will be prioritized because of their greater crossing capabilities and capacity to emit laser energy with greater fluence (80 mJ/mm2) at the maximum pulse repetition rate (80 Hz). Regarding the device settings, it is recommended to start by applying a 60 mJ/mm2 fluence and a 60 Hz pulse repetition frequency that can go up to 80 mJ/mm2 and 80 Hz based on the operator’s criterion. Energy pulses will be released while the catheter slowly moves forward through the lesion at a rate of 0.5 mm/s, thus allowing proper energy absorption and plaque modification. Retrograde application is also feasible, especially in severe lesions with antegrade resistance. Saline-infusion technique is advised. Both blood and iodinated contrast contain non-aqueous cellular macromolecules like proteins that absorb most of the energy released by the laser creating microbubbles that increase the chances of traumatic dissection.14 On the contrary, the saline solution facilitates the passage of light from the tip of the catheter to the tissue without interferences or microbubbles at that level. Therefore, the saline solution infusion technique is used to safely control the energy that is being released, and minimize the risk of dissection.15 In order to wash out the blood from the catheter-based tissue interface the catheter needs to be properly intubated and the saline solution properly infused during laser application. The application of laser to blood or contrast is allowed in selected cases of uncrossable or undilatable lesions and left to the operator’s criterion.16 At the end of the procedure, parameters like the number of pulses administered, the time of therapy, fluence, and repetition rate will need to be collected.
Crossover
Combination of several plaque modification techniques is permitted as they have shown to be complementary in some cases.17,18 If a different plaque preparation technique is required, the technique should be changed based on why the first technique failed (table 3). This switch is consistent with the routine clinical practice. All the material and techniques used will be registered for further analysis.
Table 3. Crossover of plaque modification techniques
| Failed early technique | Reason for failure | 2nd technique |
|---|---|---|
| Rotational atherectomy | Uncrossable lesion with the rotablation olive-shaped burr | ELCA |
| Undilatable lesion (suboptimal balloon expansion after rotablation) | Lithotripsy | |
| Lithotripsy | Uncrossable lesion with Shockwave balloon (despite predilatation, if necessary) | Rotational atherectomy |
| Undilatable lesion (suboptimal balloon expansion after lithotripsy) | ELCA | |
| ELCA | Uncrossable lesion with ELCA | Rotational atherectomy |
| Undilatable lesion (suboptimal balloon expansion after ELCA) | Lithotripsy | |
ELCA, excimer laser coronary angioplasty. | ||
Optical coherence tomography image acquisition and stent optimization protocol
Intravascular OCT is performed using a commercially available system (the ILUMIEN OPTIS, OPTIS Integrated, OPTIS Mobile systems, OPTISIntegrated Next, OPTISMobile Next Abbott Vascular) that incorporates a rapid exchange catheter (Dragonfly OPTIS, Dragonfly OpStar Imaging Catheter; Abbott Vascular) and an integrated pullback system (18-36 mm/s). It acquires images at high axial resolution (~15 μm) with blood displacement. A total of 3 pullbacks are advised before and after using the plaque modification technique (to describe the calcified lesion and the effects of each modality over it, respectively), and optimizing the DES implanted. The automated OCT-angiography co-registration (where available) will be used, and recommendations for PCI guidance with OCT19 will be left to the operator’s criterion. Stent expansion can be estimated using 2 methods (figure 2): 1) dual method: it identifies the stented region and splits it in half. Minimum lumen expansion in the stented area (EXP) is estimated for each half (minimum stent area in each segment divided by the proximal or distal reference area x 100). The center point can be moved by the user (both the minimum stent area and the EXP recalculate automatically); 2) tapered mode: reference lumen profile is estimated based on the distal and proximal reference frame mean diameter and side branch mean diameter in between. The software automatically displays the minimum stent area and identifies the frame with the minimum lumen expansion in the stented area (EXP). A colored expansion indicator automatically pops up when a stent is detected. Automatic detection: minimum stent area frame/Automatic detection of minimum expansion frame (EXP).
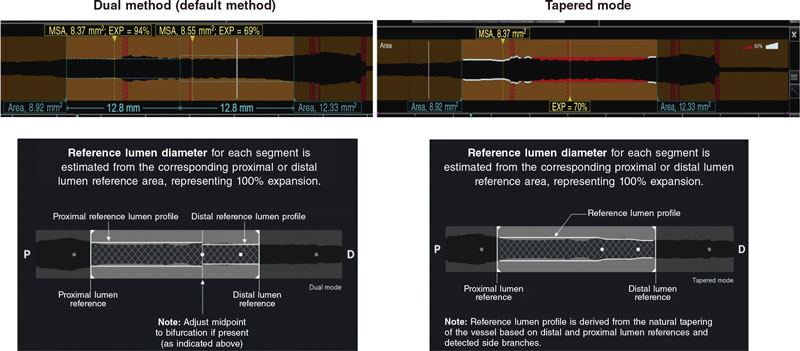
Figure 2. Stent expansion estimate by optical coherence tomography. EXP, stented area. MSA, minimum stent area. Modified with permission from Abbott Vascular from User Manual of Ultreon 1.0, and User Instructions of AptiVue Software.
With stent lengths > 50 mm, the dual method is preferred. With stent lengths < 50 mm the tapered method is often used. If the dual method is used, the stent expansion percentage of both segments is recorded being considered for analysis the lowest of the 2.
Follow-up and clinical definitions
In-hospital and follow-up outcomes were prespecified in the online database, complied with the requirements set forth by the Spanish Data Protection Act, and were only accessible to participant operators and study coordinators.
After each PCI, electrocardiographic and cardiac biomarker seriation will be performed. Clinical assessment will be conducted 1, 6, 12 months after PCI. Angiographic follow-up will be only clinically driven in patients with new symptoms, ventricular function worsening or new ischemia in non-invasive tests.
Calcification is defined as moderate if radiopacities are noted only during the cardiac cycle before contrast injection, and severe if radiopacities are noted without cardiac motion before contrast injection often compromising both sides of the arterial lumen.
Device success is defined as successful stent implantation with minimum stent areas ≥ 5.5 mm2 by OCT, final TIMI grade-3 flow, and no need for another plaque preparation strategy.
Procedural success is defined as device success and no severe procedural complications: cardiovascular death, perioperative target vessel myocardial infarction, need for new target lesion revascularization, stent thrombosis, stroke or vessel perforation with extravasation [types II or III]).
Other procedural complications included ventricular arrhythmias or hemodynamic instability during PCI, major bleeding (bleeding requiring transfusion, vasopressors, surgery or percutaneous intervention), and flow limiting dissection.
Major cardiovascular adverse events include cardiovascular death, target vessel myocardial infarction, stent thrombosis or target lesion revascularization. All deaths were considered cardiac unless other specific causes were documented. Myocardial infarction was defined according to the current recommendations made,20 and only those associated with the targer lesion, perioperative or at follow-up were considered. Target lesion revascularization or stent thrombosis were defined according to the criteria established by the Academic Research Consortium.21
Primary outcome assessment will be conducted in a central core laboratory by looking at the OCT imaging after stenting. All medical data will be codified anonymously and stored, and confidentiality will be protected at any time in observance of the current legislation. Both the clinical events committee and the independent core laboratory will be blinded to the treatment arm.
Secondary outcome assessment will be performed by assessing both the angiography and the OCT in a central core laboratory and through on-site or phone clinical follow-up sessions with the patients.
Statistical considerations
Sample size determination
This is a non-inferiority study. We expect to obtain similar outcomes regarding stent expansion using rotational atherectomy, laser, and intracoronary lithotripsy. The sample size was estimated based on the design of the trial and the results of former studies.22-24 There are no standard criteria to define stent expansion in the routine clinical practice. In a recent expert consensus document, stent expansion > 80%19 was considered appropriate. However, most former studies did not reach this threshold. In the ILUMIEN II trial, the mean stent expansion measured by OCT was 72.8% with a standard deviation of 12.6.24 To calculate the size of the sample, we assume an α error of 0.05 and a β error of 0.2 (80% power), a margin of irrelevance (ε) of 7, and losses of 10% due to measurement difficulty or impossibility to complete the intervention. With these parameters we estimate a sample size of 56 cases per group.
Statistical analysis
The study primary endpoint analysis will be conducted by lesion and intention-to-treat with a 1-sided Student t test and an alpha coefficient of 0.05 between the reference group and the other groups (ELCA, and IVL). An analysis of the primary endpoint per protocol will be conducted and presented for consistency purposes. If the hypothesis of non-inferiority is confirmed, a 2-sided superiority analysis will be conducted. Clinical endpoints will be analyzed by patient.
Quantitative variables following a normal distribution will be expressed as median ± standard deviation. Those not following such distribution will be expressed as median and minimum and maximum values. Qualitative variables will be expressed as absolute values and frequencies.
P values < .05 will be considered statistically significant, and the 95% confidence interval of the study variables will be estimated. The Kolgomorov-Smirnov test will be used to confirm the adjustment of variables to normal distribution. Regarding mean comparisons, the Student t test or the non-parametric Mann-Whitney U test (in case of qualitative dichotomous variables), and the ANOVA test or the non-parametric Kruskal Wallis test (in case of qualitative non-dichotomous variables) will be used. Regarding the bivariate analysis of qualitative variables, the chi-square test or Fisher’s exact test will be used. If necessary, the linear correlation among the different quantitative variables will be performed using Pearson correlation coefficient or Spearman’s correlation.
Regarding the multivariate analysis, the Cox regression analysis with forward, stepwise selection will be used drawing event-free survival curves using the Kaplan-Meier estimator. Variables will be considered potential predictors of risk in the multivariate model in the presence of a statistically significant correlation in the univariate analysis or a trend towards significance. The SPSS statistical software (version 20.0, SPSS Inc) will be used for all the estimates.
Organization and ethical concerns
The study protocol has been approved at each participant center by its internal ethics committee. All patients will have to give their informed written consent prior to their participation. The study is an investigator-initiated trial and follows the good clinical practice guidelines applicable to epidemiological studies. The rights and integrity of participants shall be guaranteed at all time while data confidentiality shall be safeguarded in observance of EU directives, the Declaration of Helsinki, as well as local rules and regulations. The ROLLERCOASTR trial is registered at clinicaltrials.gov wit identifier NCT04181268. The study promoter is Fundación EPIC. The study is supported by unrestricted grants from Fundación EPIC. The steering committee is the trial main decision-making committee and has final word on the medical and scientific approach to the trial. The clinical events committee includes interventional cardiologists who don’t participate in the trial and are blinded to the randomized therapy. The clinical events committee will be responsible for developing specific criteria for the adjudication of the study clinical events and endpoints as per protocol. All members of the clinical events committee will be blinded to the study primary outcomes.
DISCUSSION
At least a third of all coronary lesions requiring PCI show significant calcification.9 As a matter of fact, this is probably one of the greatest challenges interventional cardiologists face to this date. Different tools are available to prepare calcified plaques. These techniques are increasingly used in the routine clinical context based on the operator’s experience or availability25 since there are barely any comparative studies on this regard.
The role of rotational atherectomy is to facilitate stenting in calcified non-dilatable lesions. The technology has evolved for over 20 years now, and lots of patients have been treated with it. The setback is that it has a longer learning curve compared to other plaque modification techniques and requires a specific guidewire. The evidence available on RA in the calcified lesion setting shows higher procedural success rates compared to conventional or modified balloons with almost the same clinical outcomes. However, even the most recent trials have important limitations as a limited use of intracoronary imaging techniques and new-generation DES.22,23
The arrival of laser to treat atherosclerosis goes back to the 1980s to treat lower limb ischemia at the beginning, and then coronary artery disease.26 However, both catheters and the techniques were rudimentary, and complications were a common thing. The early randomized clinical trials that compared ELCA to RA or balloon angioplasty (before the stent era) did not show favorable outcomes.27 The refinement of this technology followed by the introduction of safe laser-based techniques has improved its results. However, no direct comparisons have been drawn over the past few years. Although, traditionally, severe calcification has been a non-favorable scenario for ELCA, this technique has repeatedly obtained good results in settings in which calcium is a common finding: balloon failure (uncrossable or undilatable lesions), in-stent restenosis, underexpanded stents or chronic total coronary occlusions.13 Excimer laser releases energy in the UV range in very short pulses (nanoseconds). Billions of molecules per pulse are broken. Absorption depth is 50 µm, thus reducing the risk of collateral tissue damage (compared to previous infrared lasers). Laser ablates the atherosclerotic material mediated by 3 different mechanisms: photochemical (fracture of molecular bonds): the UV light pulse hits the plaque and is highly absorbed with each photon generated carrying sufficient energy to break molecular bonds; photothermal (tissue vaporization): molecular bonds also vibrate during the absorption process resulting in heat. Intracellular water is vaporized leading to cell rupture and the creation of a vapor bubble, and photokinetic (clearance of byproducts): the rapid expansion and collapse of the vapor bubble further breaks down the plaque, but it also helps clear byproducts of ablation like water, gases, and small particles. Laser effect is amplified especially when it acts directly on blood or a contrast agent. Therefore, to reduce the risk of coronary artery dissection, laser ablation is often performed during the continuous infusion of saline solution.13 One advantage of laser is its short learning curve. It can be used through conventional 0.014 in guidewires in a rapid-exchange fashion and conventional 6-Fr guiding catheters. In addition, most of these particles are small enough to be cleared by the reticuloendothelial system, thus minimizing the risk of distal microembolization (1 more advantage compared to other plaque modification techniques).13
Lithotripsy is the latest technology that has become available to treat heavily calcified lesions. It emits pulsatile mechanical waves through emitters integrated in a semi-compliant balloon that is initially inflated at 4 atm. Afterwards, energy pulses are applied, and the vibrations produced interact with the atherosclerotic plaque breaking down both the superficial and deep calcium deposits.9 This effect on deep calcium deposits is one of the greatest advantages of lithotripsy over other techniques. Also, this technique learning curve is short since it’s based on a well-known coronary balloon technology. The DISRUPT CAD trials12 have demonstrated the safety and efficacy profile of this technique treating heavily calcified lesions and its use has grown exponentially ever since. The main limitation of this technique is that, as it is a balloon-based technology with a smaller diameter of 2.5 mm, extremely tight stenoses can hamper its use as a first-line therapy, thus needing predilatation with lower profile balloons, and even with RA17 or laser18 combined to overcome this problem.
Intracoronary imaging modalities allow more accurate assessments of coronary artery disease compared to conventional angiography and give us essential information for PCI planning. This is particularly relevant during the management of calcified and complex lesions impacting the results of the angioplasty and the patient’s prognosis28 by optimizing DES implantation, thus leading to better stent expansion, vessel wall apposition, and eventually a greater luminal area. The OCT has greater spatial resolution9 compared to the intracoronary ultrasound and has proven useful showing the effect of plaque modification therapies and stent optimization. All these reasons and the lack of use of intracoronary imaging techniques in previous plaque modification techniques has led us to using OCT to assess the study primary endpoint: percentage of stent expansion.
The ROLLERCOASTR trial will compare the 3 strategies most used in the routine clinical practice to treat lesions with moderate-to-severe calcifications. In addition, it will provide us with information on the effect of each of these strategies and the specific settings where they can be more useful. To this end, an intracoronary imaging study with an OCT will be performed to know the specific substrate of calcification and the type of plaque on which the therapy is performed as well as the effects this therapy will have. The study hypothesis is that the 3 modalities complement each other and have different effects depending on the characteristics of the lesion. At manuscript submission, a total of 135 patients have been included.
CONCLUSIONS
The ROLLERCOASTR is a prospective, multicenter, randomized clinical trial designed to compare the safety and efficacy profile of 3 plaque modification techniques in the moderate-to-severe coronary calcification setting: RA, ELCA, and IVL. The study primary endpoint is stent expansion evaluated by OCT. The secondary endpoints are device success, procedural success, crossover rate among techniques, and the occurrence of major adverse cardiovascular events at 1-year follow-up (cardiac death, target vessel myocardial infarction, need for new target lesion revascularization or stent thrombosis). We will also be describing the effects of the 3 imaging modalities in calcified lesions with OCT. Enrollment will end in 2023.
FUNDING
The study is supported by unrestricted grants from Fundación EPIC.
AUTHORS’ CONTRIBUTIONS
A. Jurado-Román: conceptualization, original draft, review, and editing. A. Gómez-Menchero, I.J. Amat-Santos, J. Caballero-Borrego, S. Ojeda, and R. Ocaranza-Sánchez: drafting, review, and editing. S. Jiménez-Valero, G. Galeote, and R. Moreno: conceptualization, drafting, review, and editing.
CONFLICTS OF INTEREST
S. Ojeda and R. Moreno are associate editors of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. S. Ojeda has received consulting fees and participated on Medtronic and Edwards Lifesciences Data Safety Monitoring Board or Advisory Boards, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript drafting or educational events organized by Philips, Biomenco, and World Medica. R. Moreno has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript drafting or educational events organized by Medtronic Inc, Boston scientific, Abbott vascular, Biosensors, Biotronik, Edwards Lifesciences, AMGEN, Astra Zeneca, Daiichi Sankyo New Vascular Therapies, and Biosensors. A. Jurado-Román has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript drafting or educational events organized by Boston Scientific, Shockwave, Philips, Biotronik, Biomenco, Abbott, and Medtronic. A. Gómez-Menchero, J. Caballero-Borrego, R. Ocaranza, G. Galeote, and S. Jiménez-Valero declared no conflicts of interest whatsoever. I. Amat-Santos has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript drafting or educational events organized by Boston Scientific.
WHAT IS KNOWN ABOUT THE TOPIC?
- Coronary calcification worsens the safety and efficacy of percutaneous coronary intervention.
- Several calcium modification techniques are currently available. However, there is a lack of randomized evidence on the therapy of choice in this scenario.
WHAT DOES THIS STUDY ADD?
- The ROLLERCOASTR is a multicenter randomized study that compared 3 advanced plaque modification techniques in the coronary calcification setting: rotational atherectomy, excimer laser, and lithotripsy.
- The study primary endpoint is stent expansion evaluated by optical coherence tomography.
- Secondary endpoints are device success (overall and depending on the type of calcific plaque), procedural success, crossover rate, and the occurrence of major adverse cardiovascular events at 1-year follow-up.
REFERENCES
1. Bourantas CV, Zhang YJ, Garg S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart Br. 2014;100:1158-1164.
2. Mori S, Yasuda S, Kataoka Y, Morii I, Kawamura A, Miyazaki S. Significant association of coronary artery calcification in stent delivery route with restenosis after sirolimus-eluting stent implantation. Circ J. 2009;73:1856-1863.
3. Tzafriri AR, Garcia-Polite F, Zani B, et al. Calcified plaque modification alters local drug delivery in the treatment of peripheral atherosclerosis. J Control Release. 2017;264:203-210.
4. Wiemer M, Butz T, Schmidt W, Schmitz KP, Horstkotte D, Langer C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc Interv. 2010;75:905-911.
5. Kobayashi Y, Okura H, Kume T, et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78:2209-2214.
6. Lee MS, Shah N. The Impact and Pathophysiologic Consequences of Coronary Artery Calcium Deposition in Percutaneous Coronary Interventions. J Invasive Cardiol. 2016;28:160-167.
7. di Mario C, Koskinas KC, Räber L. Clinical Benefit of IVUS Guidance for Coronary Stenting: The ULTIMATE Step Toward Definitive Evidence? J Am Coll Cardiol. 2018;72:3138-1341.
8. Barbato E, Shlofmitz E, Milkas A, Shlofmitz R, Azzalini L, Colombo A. State of the art: evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses - from debulking to plaque modification, a 40-year-long journey. EuroIntervention. 2017;13:696-705.
9. De Maria GL, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc Interv. 2019;12:1465-1478.
10. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
11. Barbato E, Carrié D, Dardas P, et al. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11:30-36.
12. Kereiakes DJ, Di Mario C, Riley RF, et al. Intravascular Lithotripsy for Treatment of Calcified Coronary Lesions: Patient-Level Pooled Analysis of the Disrupt CAD Studies. JACC Cardiovasc Interv. 2021;14:1337-1348.
13. Rawlins J, Din JN, Talwar S, O’Kane P. Coronary Intervention with the Excimer Laser: Review of the Technology and Outcome Data. Interv Cardiol. 2016;11:27-32.
14. Baumbach A, Haase KK, Rose C, Oberhoff M, Hanke H, Karsch KR. Formation of pressure waves during in vitro excimer laser irradiation in whole blood and the effect of dilution with contrast media and saline. Lasers Surg Med. 1994;14:3-6.
15. Tcheng JE. Saline infusion in excimer laser coronary angioplasty. Semin Interv Cardiol SIIC. 1996;1:135-41.
16. Latib A, Takagi K, Chizzola G, et al. Excimer Laser LEsion modification to expand non-dilatable stents: the ELLEMENT registry. Cardiovasc Revasc Med. 2014;15:8-12.
17. Jurado-Román A, Gonzálvez A, Galeote G, Jiménez-Valero S, Moreno R. RotaTripsy: Combination of Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Lesions. JACC Cardiovasc Interv. 2019;12:e127-129.
18. Jurado-Román A, García A, Moreno R. ELCA-Tripsy: Combination of Laser and Lithotripsy for Severely Calcified Lesions. J Invasive Cardiol. 2021;33:E754-755.
19. Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14:656-677.
20. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231-2264.
21. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-2351.
22. de Waha S, Allali A, Büttner HJ, et al. Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: Two-year clinical outcome of the randomized ROTAXUS trial. Catheter Cardiovasc Interv. 2016;87:691-700.
23. Abdel-Wahab M, Toelg R, Byrne RA, et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ Cardiovasc Interv. 2018;11:e007415.
24. Maehara A, Ben-Yehuda O, Ali Z, et al. Comparison of Stent Expansion Guided by Optical Coherence Tomography Versus Intravascular Ultrasound: The ILUMIEN II Study (Observational Study of Optical Coherence Tomography [OCT] in Patients Undergoing Fractional Flow Reserve [FFR] and Percutaneous Coronary Intervention). JACC Cardiovasc Interv. 2015;8:1704-1714.
25. Romaguera R, Ojeda S, Cruz-González I, Moreno R. Spanish Cardiac Catheterization and Coronary Intervention Registry. 30th Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2020) in the year of the COVID-19 pandemic. Rev Esp Cardiol. 2021;74:1095-1105.
26. Bittl JA, Sanborn TA, Tcheng JE, Siegel RM, Ellis SG. Clinical success, complications, and restenosis rates with excimer laser coronary angioplasty. The Percutaneous Excimer Laser Coronary Angioplasty Registry. Am J Cardiol. 1992;70:1533-1539.
27. Appelman YE, Piek JJ, Strikwerda S, et al. Randomised trial of excimer laser angioplasty versus balloon angioplasty for treatment of obstructive coronary artery disease. Lancet. 1996;347:79-84.
28. Ahn JM, Kang SJ, Yoon SH, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338-1347.
* Corresponding author.
E-mail address: alfonsojuradoroman@gmail.com (A. Jurado-Román).











