Abstract
Introduction and objectives: Recent publications suggest that bioresorbable vascular scaffolds (BVS) are associated with an excess of thrombotic complications. We present the real-world, long-term results of a series of patients who received the Absorb BVS (Abbott Vascular, United States).
Methods: A total of 213 consecutive patients who received at least 1 BVS between May 2012 and December 2016 were analyzed. The main objective of the study was the rate of target vessel failure, a composite endpoint of infarction or target vessel revascularization and cardiac death.
Results: Seventy-five per cent of the patients were men (mean age, 61.4 years). The most common cause for admission was non-ST-elevation myocardial infarction (53.52%). The median follow-up was 44 months [28 months], the rate of the primary endpoint was 6.57% for the first 24 months and 7.98% at the end of the follow-up. Regarding the device, there were 6 cases (2.81%) of thrombosis (definitive, probable or possible) and 10 cases (4.69%) of restenosis. Patients with a past medical history of diabetes mellitus (HR, 1.72; 95%CI, 1.01-2.95; P = .05) and/or chronic oral anticoagulation (HR, 5.71; 95%CI, 1.12-28.94; P = .04) had a higher risk of target vessel failure.
Conclusions: In this series of patients, the rate of target vessel failure was similar to the one previously described by randomized clinical trials. Events were more common during the first 2 years of follow-up and in the presence of greater cardiovascular comorbidity.
Keywords: Absorb. Bioresorbable scaffolds. Coronary angioplasty.
RESUMEN
Introducción y objetivos: Las publicaciones sugieren que los armazones vasculares bioabsorbibles (AVB) conllevan un exceso de complicaciones trombóticas. Se describen los resultados en la vida real y a largo plazo de una serie de pacientes a los que se implantó un AVB Absorb (Abbott Vascular, EE.UU.).
Métodos: Se analizaron 213 pacientes consecutivos que recibieron al menos un AVB entre mayo de 2012 y diciembre de 2016. El objetivo principal del estudio fue la incidencia de fracaso del vaso diana, un evento compuesto que incluye infarto de miocardio, revascularización del vaso diana y muerte cardiaca.
Resultados: El 75% de los pacientes eran varones (edad media, 61,4 años). La causa más común de ingreso fue el infarto sin elevación del ST (53,52%). La mediana de seguimiento fue de 44 meses [28 meses]. La incidencia del evento primario fue del 6,57% durante los primeros 24 meses y del 7,98% al final del seguimiento. Respecto al dispositivo, hubo 6 casos (2,81%) de trombosis (definitiva, probable o posible) y 10 casos (4,69%) de reestenosis. Los pacientes con antecedentes de diabetes mellitus (HR = 1,72; IC95%, 1,01-2,95; p = 0,05) o con anticoagulación oral crónica (HR = 5,71; IC95%, 1,12-28,94; p = 0,04) tuvieron mayor riesgo de fracaso del vaso diana.
Conclusiones: En esta serie de pacientes, la incidencia de fracaso del vaso diana fue comparable a la descrita previamente en ensayos clínicos aleatorizados. Los eventos adversos fueron más frecuentes en los primeros 2 años de seguimiento y en presencia de mayor comorbilidad cardiovascular.
Palabras clave: Absorb. Armazón vascular bioabsorbible. Angioplastia coronaria.
Abbreviations BVS: bioresorbable vascular scaffold. AMI: acute myocardial infarction. DES: drug-eluting stent.
INTRODUCTION
Drug-eluting bioresorbable vascular scaffolds (BVS) were initially presented as a technological breakthrough to overcome the limitations and adverse events associated with permanent bare-metal stents, especially the development of neoatherosclerosis that is associated with a risk of thrombosis (0.2% per year) and secondary revascularization (2% to 3% per year).1-3
At the time, the implantation of a BVS was an innovative approach to treat coronary atherosclerosis by releasing the artery from a permanent metal jail and restoring the flow architecture. Also, it preserved parietal motility and its response to stimuli generated by coronary flow (shear stress). The Absorb (Abbott Vascular, United States)—a polymer everolimus-eluting scaffold with 157 µm-thick struts—was one of the first ones to be available in Spain and several clinical trials were conducted.4-8 The excellent initial results led to the widespread use of this device for several clinical indications.9-10 The Absorb BVS was approved by the U.S. Food and Drug Administration and obtained the CE marking certification in January 2011.11
However, the mid- and long-term data of the AIDA research group12,13 on the Absorb were disappointing. They showed a higher rate of late scaffold thrombosis compared to the XIENCE (Abbott Vascular, United States) (3.5% vs 0.9%; hazard ratio [HR], 3.87; 95% confidence interval [95%CI], 1.78-8.42; P < .001), an everolimus-eluting stent (EES).14,15 Therefore, the manufacturer stopped making the Absorb BVS and removed it from the market according to the European regulatory agency; however, some of these devices remain approved and are still available in Europe.16
Since the Absorb BVS was widely used in different clinical settings during market launch more than 7 years ago, the long-term follow-up results are available today. The objective of this study is to describe the incidence of long-term adverse events in a series of patients implanted with the Absorb BVS in different clinical settings of our multicenter registry.17
METHODS
Population, design, and definitions
The cases treated with percutaneous transluminal coronary angioplasty with at least 1 Absorb BVS in 3 hospitals between May 2012 and December 2016 were studied.17 Implantation was performed to the discretion of the operator in charge.
The study primary composite endpoint was the target vessel failure rate, a composite event of target vessel revascularization, target vessel related acute myocardial infarction (AMI), and cardiac death. The study secondary endpoint was the rate of the overall clinical endpoint including these adverse events: all-cause mortality, myocardial infarction, and all the new coronary revascularizations (including those of the non-target vessel).
The registry of the interventional cardiology unit of our hospital network was periodically reviewed every 6 to 12 months at the follow-up consultation at the interventional cardiology unit by a cardiologist. Also, it was completed through follow-up phone calls.
Statistical analysis
Data regarding quantitative variables are expressed as mean ± standard deviation and qualitative variables are expressed as percentages. Patients were grouped according to whether they had target vessel failure or not; inter-group averages were compared using the Student t test. Percentages were compared using the chi-square test. Kaplan-Meier analysis was conducted to estimate the likelihood of target vessel failure-free survival and BVS thrombosis and restenosis. Finally, the multivariate Cox regression analysis was conducted to study the survival function adjusted by different predefined variables: sex, age, cardiovascular risk factors, past medical history, clinical signs, size and length of the BVS implanted, overlapping of, at least, 2 BVSs, and use of intracoronary imaging modalities (optical coherence tomography [OCT] or intravascular ultrasound [IVUS]). Two-tailed P ≤ values .05 were considered statistically significant in all tests. Data were analyzed using the statistical software package Stata IC 14 (StataCorp, United States).
RESULTS
Study population
Two hundred and thirteen consecutive patients implanted with, at least, 1 Absorb BVS between May 2012 and December 2016 were included. Table 1 shows the baseline clinical characteristics of these patients. Most of the participants were males (75.12%) with a mean age of 61.40 ± 12.74 years, and a high prevalence of dyslipidemia (62.44%) and smoking (65.26%). Diabetes mellitus was present in 23.94% and 21.60% had been previously treated with a percutaneous coronary intervention. The most common clinical presentation during recruitment was non-ST-segment elevation acute coronary syndrome (53.52%).
Table 1. Baseline clinical characteristics of patients and differences based on the primary endpoint
| Characteristics | Patients who received BVS (n = 213) | Patients with BVS and target vessel failure (n = 17) | Patients with BVS without target vessel failure (n = 196) | P |
|---|---|---|---|---|
| Age (years) | 61.40 ± 12.74 | 66.71 ± 9.62 | 61.14 ± 12.98 | .07 |
| Sex (male) | 160 (75.12) | 12 (70.59) | 148 (75.51) | .65 |
| Risk factors | ||||
| Diabetes mellitus | 51 (23.94) | 7 (41.18) | 44 (22.45) | .06 |
| Hypertension | 118 (55.40) | 11 (64.71) | 107 (54.59) | .42 |
| Dyslipidemia | 133 (62.44) | 13 (76.47) | 120 (61.22) | .21 |
| Active smoking | 139 (65.26) | 10 (58.82) | 129 (65.82) | .56 |
| Past medical history | ||||
| Chronic kidney disease | 8 (3.76) | 1 (5.88) | 7 (3.57) | .63 |
| LVEF < 30% | 5 (4.5) | 1 (5.88) | 4 (2.04) | .55 |
| Previous stroke or TIA | 9 (4.2) | 3 (17.65) | 6 (3.06) | .01 |
| Chronic oral anticoagulation | 10 (4.69) | 3 (17.65) | 7 (3.57) | .01 |
| Peripheral vascular disease | 13 (6.10) | 1 (5.88) | 12 (6.12) | .96 |
| Previous myocardial infarction | 31 (14.55) | 1 (5.88) | 30 (15.31) | .29 |
| Previous PCI | 46 (21.60) | 4 (23.53) | 42 (21.43) | .84 |
| Previous coronary artery bypass surgery | 7 (3.29) | 2 (11.76) | 5 (2.55) | .04 |
| Clinical presentation | ||||
| STEACS | 31 (14.55) | 4 (23.53) | 27 (13.78) | .25 |
| Non-Q-wave AMI type of NSTEACS | 77 (36.15) | 6 (35.29) | 71 (36.22) | .66 |
| Unstable angina type of SCASEST | 37 (17.37) | 3 (17.65) | 34 (17.35) | .88 |
| Stable angina or documented ischemia | 68 (31.4) | 4 (23.53) | 64 (32.65) | .52 |
AMI, acute myocardial infarction; BVS, bioresorbable vascular scaffold; LVEF, left ventricular ejection fraction; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome; TIA, transient ischemic attack. Data are expressed as no. (%) or mean ± standard deviation. | ||||
Index procedure of the bioresorbable vascular scaffold implantation
Table 2 shows the characteristics of the patients’ index procedure. Two hundred and thirty-three coronary lesions were treated with an average 1.3 ± 0.3 lesions per patient. Implantation was successful in 99.5% of the cases but failed in 1 patient due to the difficulty advancing the device across the lesions. The patient required the implantation of a DES, which is why he was excluded from the analysis. Predilatation occurred in 89.3% of the cases and postdilatation in 33.5% of the cases. Intracoronary imaging modalities (OCT or IVUS) were used to optimize the BVS implantation in 86 patients (40.38%).
Table 2. Characteristics of the index procedure and treatment
| Characteristics | Patients who received BVS (n = 213) |
|---|---|
| Lesions treated per patient | 1.3 ± 0.3 |
| Number of devices per patient | 1.2 ± 0.4 |
| Total length of the device per patient (mm) | 21.5 ± 13.5 |
| Minimum device diameter per patient (mm) | 2.75 ± 0.25 |
| Device implantation | |
| At least 1 BVS | 212 (99.5) |
| BVS only | 204 (95.8) |
| Overlapping with at least 2 AVBs | 20 (9.39) |
| Any DES | 8 (3.8) |
| After BVS implantation failure | 1 (0.5) |
| Procedural time (min.) | 44 ± 23 |
| Iodinated contrast used per procedure (mL) | 161 ± 72 |
| Predilatation of the first lesion treated | 189 (88.7) |
| Procedural success | 212 (99.5) |
| Lesions treated | |
| Total number | 233 |
| Predilatation | 208 (89.3) |
| Postdilatation | 78 (33.5) |
| 0.5 mm postdilatation balloon plus BVS | 21 (9.86) |
| Overall number of devices implanted | 261 |
| Overall number of devices per lesion | 1.12 ± 0.4 |
| Intracoronary imaging modality during implantation | |
| OCT or IVUS | 86 (40.38) |
BVS, bioresorbable vascular scaffold; DES, drug-eluting stent; IVUS, intravascular ultrasound; OCT, optical coherence tomography. Data are expressed as no. (%) or mean ± standard deviation. | |
Clinical follow-up
The median follow-up was 44 months [28 months] with minimum times < 1 month. The primary composite endpoint of target vessel failure rate was 6.57% at the 24-month follow-up (table 3) and 7.98% at the end of the follow-up. Figure 1 shows the target vessel failure-free survival curve; at the 48-month follow-up it was 0.92 (95%CI, 0.87-0.95; P = .02). Regarding the secondary endpoint, the overall rate was 11.74% at the 24-month follow-up (table 3) and 17.84% at the end of the follow-up.
Table 3. Adverse events at the 2-year follow-up
| Adverse event | Patients who received BVS 2-year follow-up (n = 213) |
|---|---|
| Clinical events | |
| All-cause mortality | 5 (2.34) |
| Cardiac | 3 (1.41) |
| Non-cardiac | 2 (0.94) |
| All myocardial infarctions | 6 (2.82) |
| During index procedure | 2 (0.94) |
| Not during index procedure | 4 (1.88) |
| Target vessel | 3 (1.41) |
| Non-target vessel | 1 (0.47) |
| Death or myocardial infarction | 11 (5.16) |
| Any revascularization | 18 (8.46) |
| Target vessel | 11 (5.16) |
| Target lesion | 11 (5.16) |
| Device thrombosis | 3 (1.41) |
| Device restenosis | 8 (3.76) |
| Any other vessel | 7 (3.29) |
| Composite endpoint | |
| Target vessel failure | 14 (6.57) |
| Overall clinical endpoint | 25 (11.74) |
| Device thrombosis | |
| Definite | 3 (1.41) |
| Probable | 2 (0.94) |
| Possible | 1 (0.47) |
BVS, bioresorbable vascular scaffold. Data are expressed as no. (%). | |
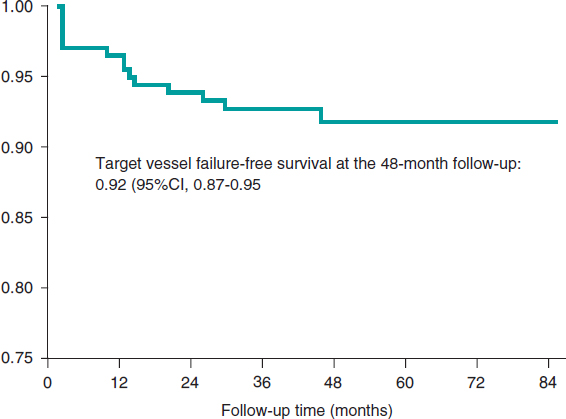
Figure 1. Kaplan-Meier survival curve for target vessel failure.
Figure 2 shows the rate of all adverse events depending on the time of clinical presentation. Regarding the primary endpoint, there were 3 (1.41%) cases of cardiac death, 4 (1.87%) cases of target vessel related AMI, and 14 (6.57%) cases of target vessel revascularization. Regarding the secondary endpoint, there were 7 (3.29%) cases of all-cause mortality, 7 (3,29%) cases of AMI, and 31 (14.56%) cases of any coronary revascularizations. Finally, regarding the device, there were 6 (2.81%) cases of thrombosis (definite, probable, and possible) all reported within the first 12 months. Dual antiplatelet therapy was kept, at least, for 12 months in 157 (73.7%) patients and 1 patient with late definite thrombosis received dual antithrombotic therapy (acenocoumarol and clopidogrel). Similarly, there were 10 (4.69%) cases of BVS restenosis within the first 48 months of follow-up (figure 3).
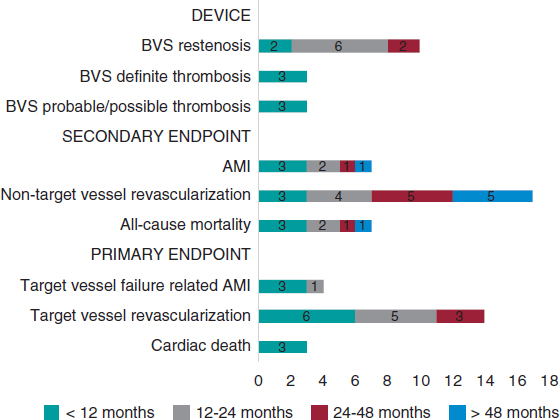
Figure 2. Chart of adverse events based on the time of presentation after the index procedure. AMI, acute myocardial infarction; BVS, bioresorbable vascular scaffold.
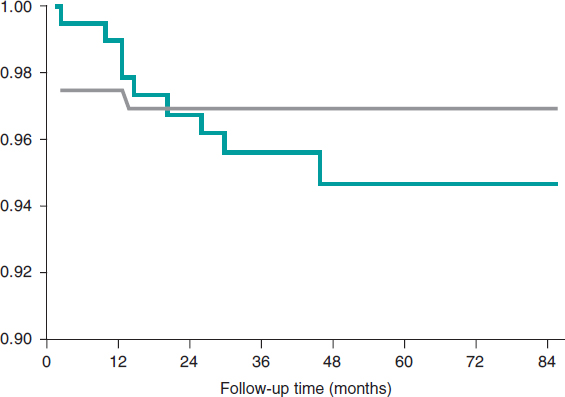
Figure 3. Kaplan-Meier survival curves for bioresorbable vascular scaffold restenosis and thrombosis.
Patients with target vessel failure had a higher prevalence of cerebrovascular disease (17.65% vs 3.06%; P = 0.01), chronic oral anticoagulation (17.65% vs 3.57%; P = .01), and previous coronary artery bypass graft surgery (11.76% vs 2.55%; P = .04). Similarly, there was a tendency towards a higher prevalence of diabetes mellitus in this group (41.18 vs 22.45%; P = .06) (table 1).
In the multivariate Cox regression analysis, a prior history of diabetes mellitus (HR, 1.72; 95%CI, 1.01-2.95; P = .05) and chronic oral anticoagulation (HR, 5.71; 95%CI, 1.12-28.94; P = .04) were identified as risk factors to develop target vessel failure at the follow-up. On the other hand, the use of intracoronary imaging modalities (OCT or IVUS) during BVS implantation showed a clear tendency towards significance as a protective factor (HR, 0.33; 95%CI, 0.10-1.07; P = .06) (table 4).
Table 4. Factors associated with target vessel failure: Cox regression analysis
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Past medical history | ||||||
| Diabetes mellitus | 1.72 | 1.04-2.86 | .04 | 1.72 | 1.01-2.95 | .05 |
| Previous stroke or TIA | 6.28 | 1.76-22.31 | .01 | 1.94 | 0.40-9.23 | .40 |
| Chronic oral anticoagulation | 5.34 | 1.51-18.97 | .01 | 5.71 | 1.12-28.95 | .04 |
| Use of intracoronary imaging modalities during implantation | ||||||
| OCT or IVUS | 0.32 | 0.11-1.03 | .06 | 0.33 | 0.10-1.06 | .06 |
95%CI, 95% confidence interval; HR, hazard ratio; IVUS, intravascular ultrasound; OCT, optical coherence tomography; TIA, transient ischemic attack. | ||||||
DISCUSSION
This study analyzed a consecutive series of patients who were implanted with, at least, 1 BVS in a high-volume setting and in real-life conditions. The primary composite endpoint of target vessel failure and the overall secondary composite clinical endpoint were similar to what had been reported by other previous randomized clinical trials on percutaneous coronary interventions.18-22
The AIDA clinical trial20 confirmed the lower rate of target vessel failure related AMI from our series. In our study, the patients’ baseline clinical characteristics and clinical presentation were similar to those of the population of the AIDA clinical trial. However, regarding the index procedure, the use of postdilatation was lower in our series. It has been reported that postdilatation does not bring any additional benefits to the implantation of a BVS in the ST-segment elevation acute coronary syndrome clinical setting. If elevation is excessive it could even have deleterious effects when destructuring or tearing the nonmetallic structure of the scaffold.23 The GHOST-EU registry24 proved that the PSP strategy (predilatation, scaffold sizing, and postdilatation) was a predictor of cardiovascular events.
The right selection of the lesion plays a crucial role in the clinical performance of BVS. Most of the patients of this series showed acute coronary syndrome. It is feasible that patients with AMI may benefit the most from BVS treatments.18 First, patients with acute coronary syndrome (with or without ST-segment elevation) often show a visible thrombus in the proximal segments and a less complex morphology with thin-cap fibroatheroma plaques and fewer calcified lesions. Secondly, aggressive antithrombotic therapy after an acute coronary syndrome may mitigate the rate of thrombotic complications.
Bioresorbable vascular scaffold thrombosis
A few studies have reported on a higher rate of BVS thrombosis associated with next-generation DESs,25,26 especially all in off-label uses.27 In our series, the definite or probable device thrombosis occurred in a similar percentage of the patients to that previously reported.12 Several mechanisms that may explain BVS thrombosis have been suggested including edge dissection, strut fracture, malapposition, and inadequate BVS sizing.28 In our series there were 2 cases of subacute definite thrombosis. In the coronary angiography, the OCT performed confirmed the presence of some structural mechanism (underexpansion or malapposition) that favored it. Early presentation at the follow-up is consistent with what has already been reported.29
Similarly, we identified that the use of intracoronary imaging modalities (OCT or IVUS) during BVS implantation showed a clear tendency towards significance as a protective factor of target vessel failure as Caixeta et al.30 had already confirmed in an international registry of 1933 patients. The recommendation here is to use intracoronary imaging modalities to optimize implantation and secure the correct apposition of the BVS, lack of underexpansion, and proper cover of the lesion.31
The main setback of the Absorb BVS is probably strut thickness and width (157 x 190.5 µm in 2.5 mm and 3.0 mm BVSs, and 157 µm x 216 µm in 3.5 mm BVSs), which can make the device more thrombogenic, especially when apposition is not the right one or expansion is incomplete. Today, ultra-thin drug-eluting stents (strut thickness < 70 µm) have lowered the risk of target lesion failure to just 1 year compared to modern second-generation DESs thanks to fewer AMIs and stent thrombosis.32 On this issue, the sirolimus-eluting MeRes100 BVS (Meril Life Sciences Pvt. Ltd., India) with thinner strut thickness (100 µm) confirmed the sustained efficacy and safety profile at the 2- and 3-year follow-up.33
Resistance to antiplatelet therapy can also be an important cause for BVS thrombosis.34 Both acetylsalicylic acid and clopidogrel are effective antiplatelet drugs for the secondary prevention of cardiovascular events. Still their clinical efficacy varies from one individual to the next.35 In our series, most of the patients remained on dual antiplatelet therapy for, at least, 12 months and there was 1 case of late thrombosis with dual antithrombotic therapy (acenocoumarol and clopidogrel). Due to his high bleeding risk, this last patient received dual antiplatelet therapy for the first 3 months; we do not know the international normalized ratio when the complication occurred, which is why the possibility of antiplatelet drug resistance cannot be discarded. However, the potential association between the BVS thrombosis and oral antiplatelet therapy had already been described.36 We know that the selection of duration of antiplatelet therapy following the implantation of the Absorb BVS was difficult,37 especially in anticoagulated patients because they are a population with comorbidities and high cardiovascular risk. Our data show that the implantation of the Absorb BVS in patients at high bleeding risk (including anticoagulated patients) shouldn’t probably be recommended according to the consensus document reached by the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery. This document does not recommend the use of the Absorb BVS in patients intolerant to prolonged dual antiplatelet therapy or who require oral anticoagulation.16
Bioresorbable vascular scaffold restenosis
The most common cause for target lesion revascularization was stent restenosis within the first 48 months of follow-up. The mechanisms involved in bioresorbable vascular scaffold restenosis that may occur in the same patient are varied.38,39 The less intrinsic radial strength and its possible destructuring with an aggressive implantation may explain some of the early recurrences. In this study, aggressive implantation was less common since postdilatation with an up to 0.5 mm balloon combined with BVS implantation occurred in 9.86% of the cases. Also, postdilatation was not associated with restenosis at the follow-up. Also, it has been suggested that the slow resorption of the study device may have been associated with a significant spatial abnormality with loss of alignment of its structural elements, which favors restenosis.40,41 The complete disappearance of the BVS from the vascular wall won’t happen for another 3 years6 and most cases of scaffold restenosis occurred within the first 2 years of follow-up.
Our study results show that there is a correlation between the history of diabetes mellitus and chronic oral anticoagulation and the development of target vessel failure. It is well-known that this past medical history elevates cardiovascular morbimortality and that the CHADS2 and CHA2DS2-VASc scores can be used to estimate the risk of adverse clinical events in patients with acute coronary syndrome.42 In this sense, patients with a past medical history of diabetes mellitus, chronic oral anticoagulation, and coronary artery disease start with CHA2DS2-VASc scores of 4, that is, high risk of adverse clinical events.
Limitations
Selection bias was inevitable because, according to the operator’s criterion, the clinical assessment that may have influenced the decision to implant a BVS maybe did not come from the database, which is a common problem with observational studies like this one. However, the study shows a pragmatic approach to the use of this device in the real world.
CONCLUSIONS
In this series of patients implanted with the Absorb BVS, the composite endpoint of target vessel failure and the overall clinical composite endpoint were similar to what had already been reported by randomized clinical trials. Adverse events were more common within the first 2 years of follow-up in case of greater cardiovascular comorbidity and without intracoronary imaging modalities (OCT or IVUS) during implantation. Although the BVS studied is not available anymore there other bioresorbable devices are in the pipeline.16
FUNDING
R. Mori-Junco received the 2018 training grant from the European Society of Cardiology (APP000019660). L. Furuya-Kanamori received funding from the Australian National Health and Medical Research Council Early Career Fellowships (APP1158469).
CONFLICTS OF INTEREST
The authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- The implantation of a BVS is an innovative approach for the management of coronary atherosclerosis because it releases the coronary artery from a permanent metallic jail and restores the vessel architecture.
- However, the Absorb BVS has a higher rate of thrombotic complications compared to modern DESs, which is why it was removed.
WHAT DOES THIS STUDY ADD?
- In our interventional cardiology network, the implantation of the Absorb BVS showed rates of target vessel failure that were similar to those previously described by randomized clinical trials.
- Target vessel failure occurred basically within the first 24 months in patients with diabetes mellitus or chronic oral anticoagulation. The use of intracoronary imaging modalities during implantation showed a tendency towards becoming a protective factor.
- Our results will contribute to the proper selection of patients eligible for BVS implantation and to the implantation technique as well.
REFERENCES
1. Smits PC, Vlachojannis GJ, McFadden EP, et al. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice:The COMPARE Trial (A Trial of Everolimus-Eluting Stents and Paclitaxel Stents for Coronary Revas.cularization in Daily Practice). JACC Cardiovasc Interv. 2015;8:1157-1165.
2. Byrne RA, Stone GW, Ormiston J, Kastrati A. Coronary balloon angioplasty, stents, and scaffolds. Lancet. 2017;390:781-792.
3. Ellis SG, Riaz H. Bioresorbable stents:The future of interventional cardiology?Cleve Clin J Med. 2016;83:S18-S23.
4. Ellis SG, Kereiakes DJ, Metzger DC, et al. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med. 2015;373:1905-1915.
5. Ormiston JA, Serruys PW, Regar E, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB):a prospective open-label trial. Lancet. 2008;371:899-907.
6. Serruys PW, Garcia-Garcia HM, Onuma Y. From metallic cages to transient bioresorbable scaffolds:change in paradigm of coronary revascularization in the upcoming decade?Eur Heart J. 2012;33:16-25.
7. Serruys PW, Katagiri Y, Sotomi Y, et al. Arterial Remodeling After Biore.sorbable Scaffolds and Metallic Stents. J Am Coll Cardiol. 2017;70:60-74.
8. Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolim.us-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II):a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479-2491.
9. Rampat R, Mayo T, Hildick-Smith D, Cockburn J. A randomized trial comparing two stent sizing strategies in coronary bifurcation treatment with bioresorbable vascular scaffolds –The Absorb Bifurcation Coronary (ABC) trial. Cardiovasc Revascularization Med. 2019;20:43-49.
10. Mitomo S, Naganuma T, Fujino Y, et al. Bioresorbable Vascular Scaffolds for the Treatment of Chronic Total Occlusions. Circ Cardiovasc Interv. 2017;10:e004265.
11. Abbott Laboratories. Abbott Receives CE Mark Approval for World's First Drug Eluting Bioresorbable Vascular Scaffold for Treatment of Coronary Artery Disease. Available online:https://www.prnewswire.com/news-releases/abbott-receives-ce-mark-approval-for-worlds-first-drug-eluting-bioresorbable-vascular-scaffold-for-treatment-of-coronary-artery-disease-113197364.html. Accessed 25 Aug 2019.
12. Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med. 2017;376:2319-2328.
13. Kerkmeijer LSM, Tijssen RYG, Hofma SH, et al. Comparison of an evero.limus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent in routine PCI:three-year clinical outcomes from the AIDA trial. EuroIntervention. 2019;15:603-606.
14. Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease:a patient-level, pooled meta-analysis. Lancet. 2016;387:1277-1289.
15. Katsikis A, Serruys PW. Bioresorbable scaffolds versus metallic stents in routine PCI:the plot thickens. J Thorac Dis. 2017;9:2296-2300.
16. Byrne RA, Stefanini GG, Capodanno D, et al. Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention:executive summary. Eur Heart J. 2018;39:1591-1601.
17. Nuñez Gil IJ, Bas M, Fernández-Ortiz A, et al. Long term experience with a novel interventional cardiology network model:Learned lessons. J Hosp Adm. 2016;5:87-94.
18. Byrne RA, Alfonso F, Schneider S, et al. Prospective, randomized trial of bioresorbable scaffolds vs. everolimus-eluting stents in patients undergoing coronary stenting for myocardial infarction:The Intracoronary Scaffold Assessment a Randomized evaluation of Absorb in Myocardial Infarction (ISAR-Absorb MI) trial. Eur Heart J. 2019;40:167-176.
19. Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice:Early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015;10:1144-1153.
20. Tijssen RYG, Kraak RP, Hofma SH, et al. Complete two-year follow-up with formal non-inferiority testing on primary outcomes of the AIDA trial comparing the Absorb bioresorbable scaffold with the XIENCE drug-eluting metallic stent in routine PCI. EuroIntervention. 2018;14:e426-e433.
21. Chevalier B, Onuma Y, Boven AJ Van. Randomised comparison of a bioresorbable everolimus- eluting scaffold with a metallic everolimus-eluting stent for ischaemic heart disease caused by de novo native coronary artery lesions:the 2-year clinical outcomes of the ABSORB II trial. EuroIntervention. 2016;12:1102-1107.
22. Alvarez M, Applegate RJ. Early and Late Bioresorbable Vascular Scaffold Thrombosis:Size Matters. JACC Cardiovasc Interv. 2017;10:2372-2374.
23. Yamaji K, Brugaletta S, SabatéM, et al. Effect of Post-Dilatation Following Primary PCI With Everolimus-Eluting Bioresorbable Scaffold Versus Everolimus-Eluting Metallic Stent Implantation. JACC Cardiovasc Interv. 2017;10:1867-1877.
24. Ortega-Paz L, Capodanno D, Gori T, et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events:Development and internal validation of the PSP score. EuroIntervention. 2017;12:2110-2117.
25. Lipinski MJ, Escarcega RO, Baker NC, et al. Scaffold Thrombosis After Percutaneous Coronary Intervention With ABSORB Bioresorbable Vascular Scaffold. JACC Cardiovasc Interv. 2016;9:12-24.
26. Alfonso F, Cuesta J. Very Late Bioresorbable Vascular Scaffold Thrombosis:Smoke or Fire?JACC Cardiovasc Interv. 2017;10:38-41.
27. Miyazaki T, Ruparelia N, Kawamoto H, Figini F, Latib A, Colombo A. Clinical outcomes following “off-label“versus “established“indications of bioresorbable scaffolds for the treatment of coronary artery disease in a real-world population. EuroIntervention. 2016;11:1475-1478.
28. Puricel S, Cuculi F, Weissner M, et al. Bioresorbable Coronary Scaffold Thrombosis. J Am Coll Cardiol. 2016;67:921-931.
29. Brugaletta S, Gori T, Low AF, et al. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction:1-year results of a propensity score matching comparison:the BVS-EXAMINATION Study (bioresorbable vascular scaffold - a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv. 2015;8:189-197.
30. Caixeta A, Campos CM, Felix C, et al. Predictors of long-term adverse events after Absorb bioresorbable vascular scaffold implantation:a 1,933.patient pooled analysis from international registries. EuroIntervention. 2019;15:623-630.
31. IJsselmuiden AJJ, Zwaan EM, Oemrawsingh RM, et al. Appropriate use criteria for optical coherence tomography guidance in percutaneous coronary interventions:Recommendations of the working group of interven.tional cardiology of the Netherlands Society of Cardiology. Neth Heart J. 2018;26:473-483.
32. Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer-Generation Ultra-thin Strut Drug-Eluting Stents Versus Older Second-Generation Thicker Strut Drug-Eluting Stents for Coronary Artery Disease. Circulation. 2018;138:2216-2226.
33. Seth A, Onuma Y, Chandra P, et al. Three-year clinical and two-year multimodality imaging outcomes of a thin-strut sirolimus-eluting bioresorb.able vascular scaffold:MeRes-1 trial. EuroIntervention. 2019;15:607-614.
34. Fernández-Rodríguez D, Brugaletta S, Otsuki S, SabatéM. Acute Absorb bioresorbable vascular scaffold thrombosis in ST-segment elevation myocardial infarction:to stent or not to stent?EuroIntervention. 2014;10:600;discussion 600.
35. Tantry US, Navarese EP, Bliden KP, Gurbel PA. Acetylsalicylic acid and clopidogrel hyporesponsiveness following acute coronary syndromes. Kardiol Pol. 2018;76:1312-1319.
36. Cayla G, Koning R, Fajadet J, et al. Percutaneous coronary interventions with the Absorb Bioresorbable vascular scaffold in real life:1-year results from the FRANCE ABSORB registry. Arch Cardiovasc Dis. 2019;112:113-123.
37. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J. 2018;39:213-260.
38. Núñez-Gil IJ, Echavarría M, Escaned J, Biagioni C, Feltes G, Fernández-Ortiz A. Bioresorbable stent restenosis:new devices, novel situations. J Invasive Cardiol. 2014;26:E164-6.
39. Longo G, Granata F, Capodanno D, et al. Anatomical features and manageµment of bioresorbable vascular scaffolds failure:A case series from the GHOST registry. Catheter Cardiovasc Interv. 2015;85:1150-1161.
40. Nakatani S, Onuma Y, Ishibashi Y, et al. Early (before 6 months), late (6-12 months) and very late (after 12 months) angiographic scaffold restenosis in the ABSORB Cohort B trial. EuroIntervention. 2015;10:1288-1298.
41. Räber L, Brugaletta S, Yamaji K, et al. Very Late Scaffold Thrombosis. J Am Coll Cardiol. 2015;66:1901-1914.
42. Chua S-K, Lo H-M, Chiu C-Z, Shyu K-G. Use of CHADS2 and CHA2DS2.VASc scores to predict subsequent myocardial infarction, stroke, and death in patients with acute coronary syndrome:data from Taiwan acute coro.nary syndrome full spectrum registry. PLoS One. 2014;9:e111167.
Corresponding author: Instituto Cardiovascular, Cardiología Intervencionista, Hospital Clínico San Carlos, Profesor Martín Lagos s/n, 28040 Madrid, Spain.
E-mail address: ibnsky@yahoo.es (J. Núñez Gil).











