Innovation in interventional cardiology
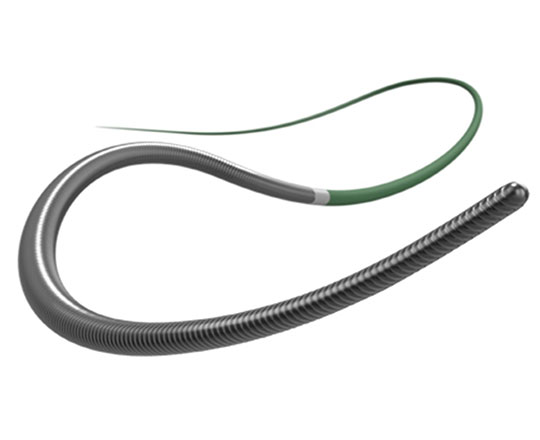
New to the market is the Hi-Torque Infiltrac guide wire (Abbott, United States), a new angioplasty guide wire with high penetration power for the treatment of chronic occlusions.
It is a tapered (0.009'') guide wire made of hydrophilic-coated Durasteel stainless steel, providing better lubricity and facilitating movement within the microcatheter. The distal 3 cm are radio-opaque, and the tip does not have a hydrophilic-coating to offer better control and tactile feedback.
The Hi-Torque Infiltrac guide wire’s special tip technology has great penetration power thanks to its unique microtexture design, which provides greater feedback and safety during procedures. Another key characteristic is the guide wire’s pre-formed 1-mm Micro-J tip with 25° angulation, which provides steerability and facilitates access to the occlusion without causing prolapse.
The guide wire is available in two tip loads and penetration powers to facilitate access to all types of occlusion: Hi-Torque Infiltrac, at 11 g and 167 kg/in2, and Hi-Torque Infiltrac Plus, at 14 g and 224 kg/in².
Given its design, it looks to be a candidate for antegrade wire escalation in cases with a proximal capsule resistant to penetration.
Keywords: percutaneous coronary intervention, coronary guidewire, coronary chronic total occlusion.

The Perclose ProStyle system (Abbott Vascular Inc, United States) is the newest generation of the Perclose vascular closure family, designed to percutaneously apply a suture to close the femoral artery or vein access site in patients undergoing diagnostic or interventional catheterization procedures. We could describe its function as a vascular closure system that, as well as closing the access site, repairs it.
Hemostasis is achieved by approximating the edges of the vessel wall with a biocompatible surgical suture to promote healing by primary intention,1 leaving smaller scars,2 allowing immediate re-access, and reducing the time to ambulation and hospital discharge.3
This new system comprises three elements that help the user to position, approximate, tie off and then cut the remaining ends of the suture:
- A Perclose ProStyle device designed to ensure greater needle resistance and ease of use.
- The device sheath contains a large, white, guide-wire exit port for better visibility and easier guide wire insertion. The proximal, metal part of the guide wire has reference markers that provide a visual estimation of the depth of the subcutaneous tract during suture deployment. The distal, hydrophilic-coated guide wire allows smoother advancement of the device and increases needle resistance, to minimize deviation in thickened tissues. The plunger has an improved spring to ensure better-controlled placement of the needles at the foot without a rebound affect.
- A suture trimmer and a snare-knot pusher, designed to approximate the knot to the top part of the access site. The suture trimmer has a metallic, hydrophilic-coated sheath, depth reference markers that line up with those of the device, and a more rigid cutting lever to reduce the possibilities of accidental cutting. The snare-knot pusher has been included as an additional low-profile accessory to facilitate advancing the knot in cases of deeper access.
BIBLIOGRAFÍA
1. FDA Databases. Perclose™ ProStyle™ SMCR System - Instructions for Use. Available at https://fda.report/GUDID/28717648235188. Accessed 10 Apr 2021.
2. Mercandetti, M. Wound Healing and Repair. Medscape. 02 Apr 2019. Available at: https://emedicine.medscape.com/article/1298129-overview. Accessed 10 Apr 2021.
3. Bhatt DL, Raymond RE, Feldman T, et al. Successful "pre-closure" of 7Fr and 8Fr femoral arteriotomies with a 6Fr suture-based device (the Multicenter Interventional Closer Registry). Am J Cardiol. 2002;89:777-779.
Keywords: percutaneous vascular closure device.
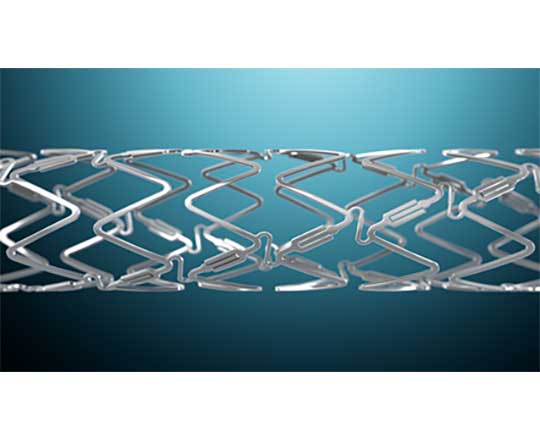
The DynamX Bioadaptor (Elixir Medical Corporation, United States), is a novolimus-eluting stent with a bioresorbable polymer coating. However, the stent has a unique hybrid design, with metallic segments that fit together with resorbable polymer connections.
Once implanted, the stent’s resorbable polymer elutes the drug (novolimus) for the first 3 months, and the polymer and connections between the metallic parts resorb over the next six months. This process uncages some elements of the stent in the artery and the rigid metallic structure is freed, allowing a more physiological positive adaptive remodeling of the vessels. This restores the pulsatile movement of the vessel and the physiological cyclical pressure and returns the artery to its original angulation so that it can move with the natural expansion and contraction, unlike a conventional metallic stent.
The first patient has just been enrolled in the BIOADAPTOR study, a multicenter, single-blind randomized clinical trial that will enroll 444 patients (mainly in Japan and Germany) treated with DynamX Bioadaptor with a 1:1 randomization versus Resolute Onyx (Medtronic, United States). It will be very interesting to see if there is any place for resorbable technology in coronary metallic stents.
Keywords: percutaneous coronary intervention, drug-eluting stent.
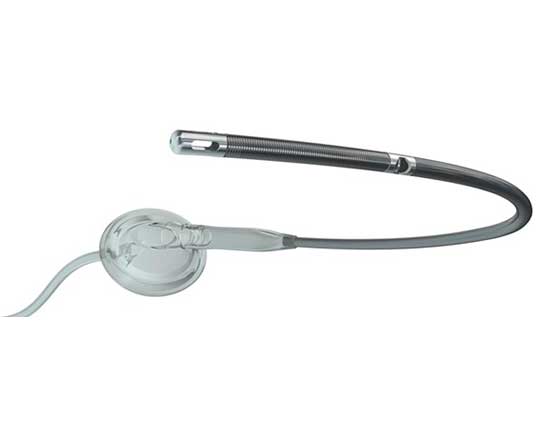
PulseCath iVAC2L (Terumo, Japan), is a percutaneous pulsatile pump for use as short-term mechanical circulatory support. It currently has the CE mark for use in high-risk coronary interventions, but is not indicated for use in cardiogenic shock. After transfemoral implantation via an 18-French sheath, this device increases cardiac output up to 2 L/min, maintaining sufficient coronary and vital organ perfusion and reducing the ventricular systolic workload.
Unlike other mechanical devices that use a continuous flow or axial pump, the iVAC2L has the advantage of being suitable to combine with any counterpulsation balloon console. As it does not need a special console, its operation is simplified and its costs are reduced. Another advantage is that it generates a more physiological pulsatile flow that is less deleterious for the patient.
The helium is connected to an external, transparent, flexible-membrane pump and in turn to the catheter. The catheter has a two-way valve 6 cm from the distal tip of the catheter, where there is an inlet. During systole, blood is aspirated from the left ventricle toward the membrane pump, and during diastole it is expelled toward the ascending aorta via a lateral outlet in the catheter. This process is synchronized with the cardiac cycle thanks to the counterpulsation balloon, reducing the systolic load and oxygen demand. Electrocardiography or the pressure waveform trigger the moment at which the helium enters the membrane pump, synchronizing the pump with the heart, and preserving neurohumoral pathways better than other mechanical support systems.
The device causes minimal hemolysis, similar to other devices already on the market.
An important disadvantage is its current requirement for 18-French femoral access, compared with the 12-French, and possibility of single access, offered by the competition.
Keywords: coronary disease, heart failure, high-risk percutaneous coronary intervention, mechanical circulatory support.
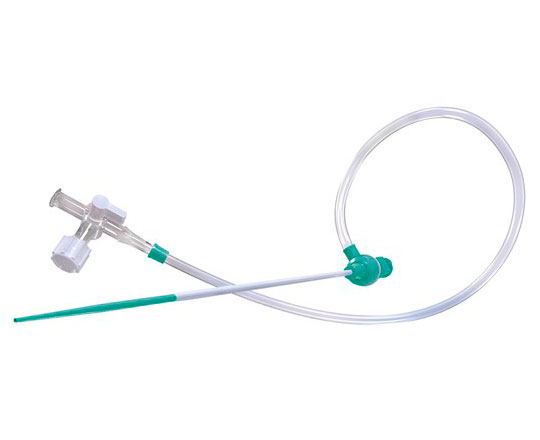
In certain fields, the high quality and excellence of existing materials make continued innovation difficult. Transradial introducer sheaths represent one such field.
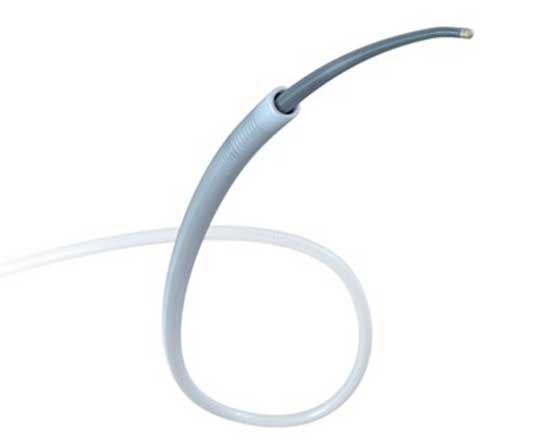
In 2020, Cardinal Health (USA) presented the RAIN Sheath vascular introducer. This device has an ultrafine wall that allows it to have an outer diameter that is one French smaller than that of the catheter that will be passed through it. For example, for a 6-French catheter, the outer profile of the introducer is equivalent to that of a 5-French. It is made with a polymer that has elastomeric qualities that provide great flexibility and better resistance to torsion than other similar devices on the market. Thin-walled introducers are prone to torsion and lumen collapse, but this is avoided thanks to the properties of the RAIN Sheath.
One of the key innovations is that it is available in 4, 5, 6 and 7 French, for cases in which the radial artery is very small caliber but radial access is nonetheless required. Other details are its high-performance hydrophilic coating, which facilitates atraumatic entry and withdrawal, as well as a hexacuspid valve that prevents bleedback.
As with other commonly-used introducers, it comes in a kit with a 45 cm, 0.021’’ diameter metal or polymer guidewire and a 21 G bare needle or IV catheter needle.
Palabras clave: intervención coronaria percutánea, introductor vascular, acceso radial. Keywords: percutaneous coronary intervention, vascular sheath, radial access.

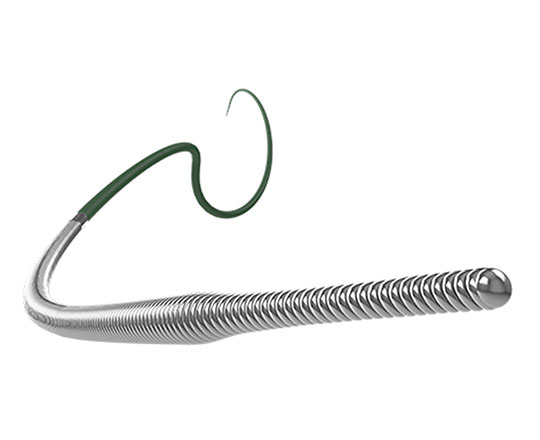
A new coronary angioplasty guidewire has been brought to the North American market. This hybrid guidewire combines several technologies. It is produced by the company Asahi Intecc (Japan), which, in my opinion, is a guarantee of success. It is called Minamo and it has a three-piece coaxial core. These pieces are, from proximal to distal: a round stainless steel core, followed by a tapered nitinol core, and most distally, a stainless steel round press core. This end segment is surrounded by a 170 mm coil, with the most distal 30 mm containing braided wires with ACT One technology. As is well known, this section with ACT One technology allows the preshaped curve to retain its shape for a longer time and be reshaped as many times as necessary without breaking the guidewire. It can be preshaped easily with a special needle, and even it this is done several times, it forms a perfect J curve without distortion.
The Minamo guide wire is suitable for everyday use in most interventional procedures. It has a tip load of 0.5 gf. The most distal 25 mm of the tip is silicone-coated, and the remainder is hydrophilic-coated.
Palabras clave: intervención coronaria percutánea, guía de angioplastia coronaria. Keywords: percutaneous coronary intervention, coronary wire.

The practice of working with robots has been incorporated for some time in many surgical specialties. In cardiac catheterization, great advances in the development of devices have allowed us to treat cardiac conditions that would previously have been unthinkable. The way we perform catheterization has not changed much since 1958, when the first angiography was performed, and physicians are still exposed to disease-causing radiation and have to tolerate the weight of lead aprons. Robots allow us to perform an intervention on a patient, not only from a physical distance, but also a geographical distance. This could be a solution to the problem of certain acute patients accessing – in a timely manner – centers with interventional facilities (24 hours a day, 7 days a week, 365 days a year).
A very advanced system, from Corindus (recently acquired by Siemens) has been around for years, undergoing frequent improvements. Another, lesser-known system is also available – Robocath1.
This system has two key components. The first is an articulated arm that holds a device where the guide catheter, angioplasty guidewire, and balloons or stents are placed along with the connections to the infusion pump and saline flush. The second is a mobile control unit, which contains the screens, two joysticks – one to steer the catheter and one for the guidewire – the control for contrast injection and the controls for the table.
The aim of robotic assistance in cardiac catheterization is to perform the procedures safely, comfortably, and accurately, working in the best interests of the patients. It is clear that telemedicine will undergo substantial development in the coming years, as we have seen during 2020 with the COVID-19 pandemic. The problem with robotic assistance in cardiac catheterization is that currently it can be used for simple cases, but a lot of fine tuning is still needed for more complex cases and complications that may arise during procedures. It is a very interesting area of innovation that has a lot of future potential.
BIBLIOGRAFÍA
1. Robocath. 2021. Available at https://www.robocath.com/. Accessed 4 Jan 2021.
Palabras clave: intervención coronaria percutánea, asistencia robótica. Keywords: percutaneous coronary intervention, robotic-assisted.
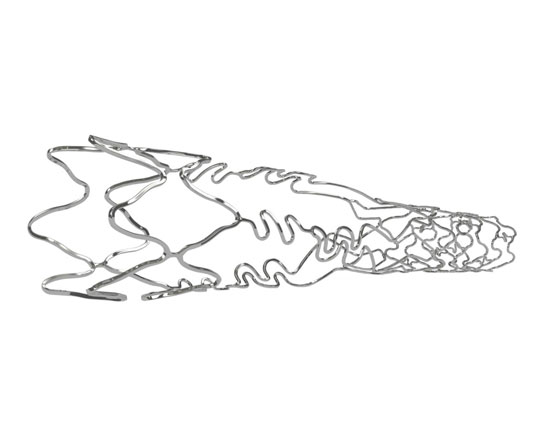
Older devices can be revived with innovative technological improvements. This is the case for the design of a stent specifically for bifurcations, now called Biomime Branch (Meril, India).
This is a chrome-cobalt stent with 65 µm strut thickness. It is covered with a resorbable PLGA-PLLA polymer that elutes sirolimus at a dose of 1.25 µg/mm2. The device’s architecture is interesting because it has a hybrid design, which is very useful for treating 1,1,1 bifurcations in which there is a very high probability of needing more than one stent. It is mounted on a balloon (with four radio-opaque markers) with a tapered design, which allows stent implantation from the main branch into the side branch. The proximal segment (which is positioned in the main branch) has a closed-cell design and the distal segment (for the side branch lesion) has an open-cell design. The distal segment has a variable length, from 6.5 to 13 mm, to ensure coverage of side branch lesions.
Between the two segments there is a transition zone with three panels with highly flexible connectors that leave large open spaces, allowing access to the main branch to implant a further stent without the risk of losing the side branch, and avoiding overexpansion of the stent at post-dilatation. This reduces the risk of losing important branches with severe lesions.
In my opinion, this is a very interesting device, as it simplifies the procedure, reduces the intervention time when it is likely that 2 stents will be implanted, and limits the amount of metal that may be exposed at the carina.
Palabras clave: Intervención coronaria percutánea, bifurcación coronaria, stent coronario para bifurcación, stent coronario liberador de droga. Keywords: Percutaneous coronary intervention, coronary bifurcation, bifurcation dedicated stent, drug eluting stent.