Innovation in interventional cardiology
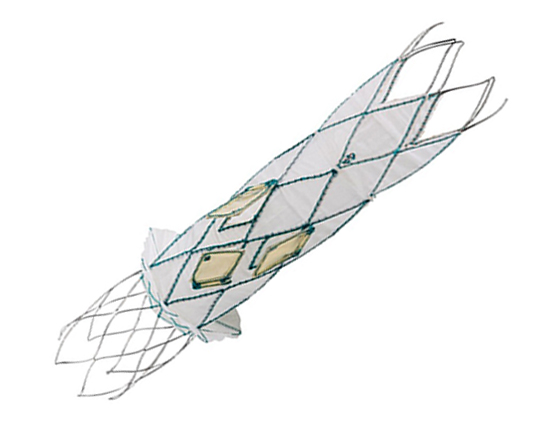
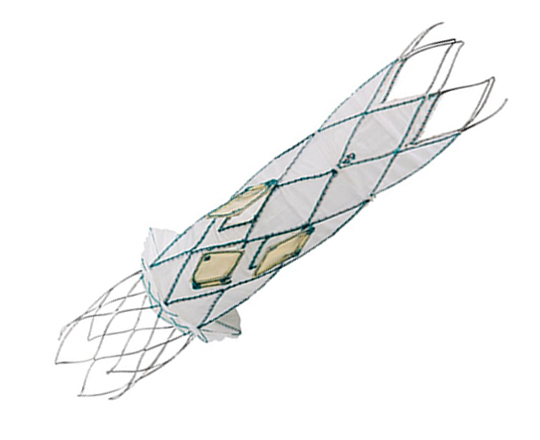
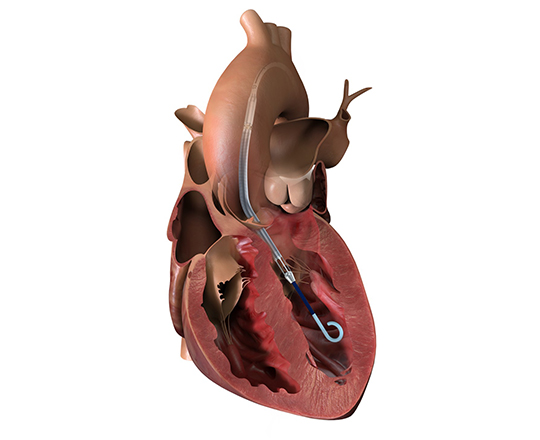
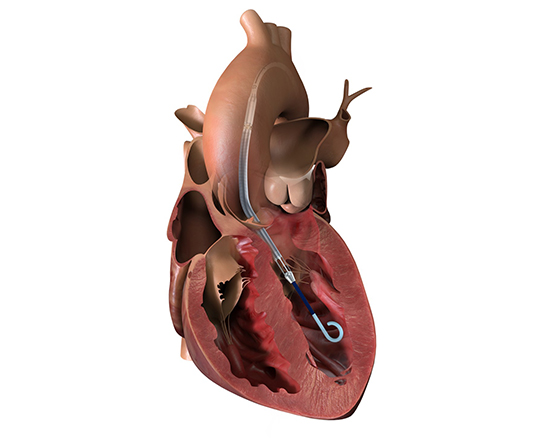
The Trillium device (Innoventric, Israel) is an endoprosthesis for tricuspid regurgitation that is positioned to run from the superior vena cava to the inferior vena cava. It has multiple covered fenestrations, which allow the inflow of blood from the venous system toward the right atrium through three windows, avoiding backflow to the venous system. It comes with a 24 Fr deployment system.
The prosthesis has fluoroscopic markers along its structure to facilitate its positioning. The procedure is strictly fluoroscopic and is performed via transfemoral venous access.
The cylindrical shape of the device facilitates its positioning it in the right atrium, as it can rotate without limiting the valves’ opening, providing greater safety to this novel technology. The Trillium valve system includes a distal skirt, designed to block inferior vena cava backflow without blocking inflow from the hepatic vein in patients with a very short distance between the hepatic veins and the right atrial cavity.
It can be positioned on top of a previously-implanted pacemaker and it can be used along with implantable cardioverter-defibrillator leads.
Currently, the Trillium system is undergoing its first-in-human clinical trial (NCT 04289870), with more than 10 patients treated in Germany, Belgium, and Israel to date. In early 2022, it began to be used in Spain and other EU countries as part of its clinical development to obtain the CE mark. There are very high expectations for this valve.
Keywords: Tricuspid regurgitation, percutaneous prosthetic valve.
A new generation of Impella (Abiomed, USA) has just arrived on the market: the Smart Assist (SA) model, more functional and adapted to the new technological era in which we live. The sensor type and position have been changed: it now has an optical sensor in the blood outflow cannula, which allows more precise confirmation of the position of the device and reduces the risk of hemolysis. It also provides better hemodynamic support, with peak flows of 4.3 L/min (mean flow, 3.8-3.9 L/min) for the Impella SA (up to 46 000 rpm support, with no time limit). All this hemodynamic information is available on the controller display screen, which shows the left ventricular pressure, the total cardiac output (Impella flow + native cardiac output) and the CPO (cardiac power output).
The aspiration and position alarms are displayed more quickly and precisely, as is their resolution. The pump can be positioned without imaging using the controller.
Furthermore, the screen displays trends that help guide the patient management during support and weaning, simplifying decision-making.
The set-up is faster, as it has fewer connections (it has a lead that is already set-up, and the red pressure sensor light has been removed).
The introducer kit contains, in addition to the 14 Fr x 13 cm introducer, another 14 Fr x 25 cm introducer with longer, tapered dilators.
Soon, the platform for remote access to the controller using artificial intelligence, called Impella Connect, will be available—a great technological advance.
Keywords: High-risk PCI, Impella SA, cardiogenic shock, heart assist devices, hemodynamics, mechanical support devices.
The Ostial Flash balloon (Cardinal Health, USA) has a dual-balloon design to conform to the coronary ostium during postdilatation of stents implanted in this area. We know how difficult it can be to implant a stent fully adapted to the ostium to provide good coverage of the proximal plaque, especially in aorto-ostial lesions, where there is almost always a proximal portion of the stent that protrudes from the orifice, with the difficulties that can arise in subsequent recatheterizations, as the catheter can distort the proximal part of the stent and may even pass through the lateral struts in the treatment of potential new lesions.
The balloon has 3 markers: the two most distal are on the distal and proximal ends of the implanted stent and the most proximal marker lies outside the stent, in the aorta. First, the distal balloon is inflated using the indeflator, then the proximal, compliant balloon is inflated at low pressure using a 1-cc syringe. In this way, the protruding area remains conformed to the aortic wall, avoiding future reaccess problems if required.
This is a very useful device for aortocoronary lesions of the native bed and aortocoronary grafts, as it ensures perfect stent positioning and apposition. It also comes in larger sizes for peripheral vessels. It is available in diameters of 3.0 to 4.5 mm and lengths of 8 and 12 mm.
Keywords: Aorto-ostial coronary lesions, stent postdilatation.
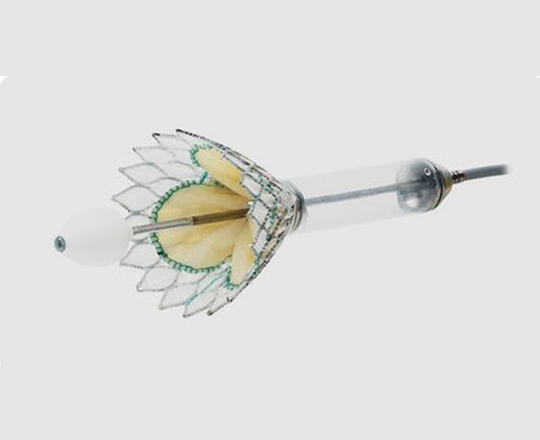
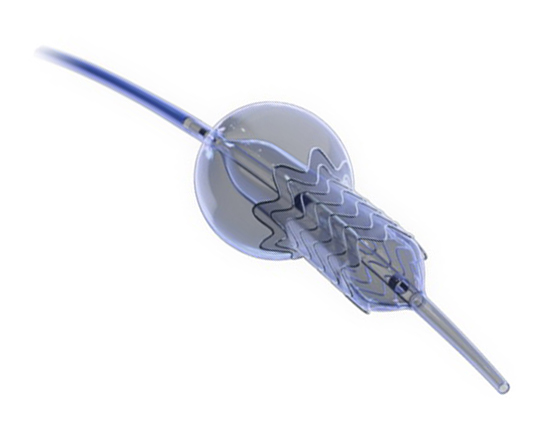
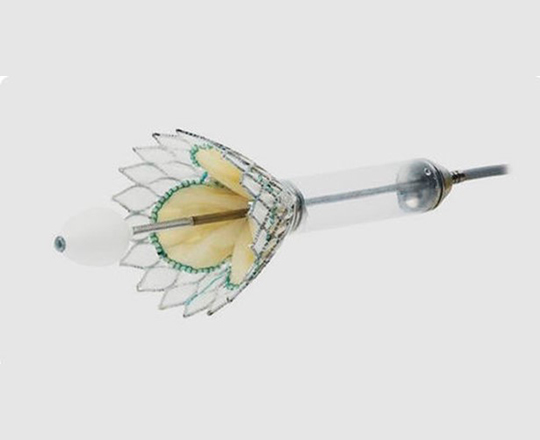
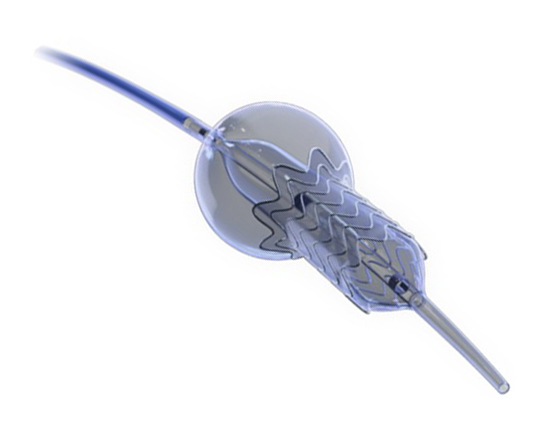
Introducing an atrioventricular valve indicated for transcatheter implantation in cases of severe mitral regurgitation, particularly functional regurgitation (it is also valid for use in some cases of mixed degenerative disease and even focal calcification). Cardiovalve (Venus Medtech, China) has a height of 15 mm to avoid left ventricular outflow tract obstruction, something that is common with other valves. It is made with a double metal frame formed by a stent where the valve is sewn, and an (inflatable) skirt that adapts to the annulus to avoid paravalvular leaks. It is available in three sizes: medium, large, and extra large. The medium covers annulus diameters of between 36 and 43 mm; large, between 42 and 48 mm; and extra large, from 47 to 53 mm. The same valve can be used to treat tricuspid regurgitation: for the moment, it is only indicated in patients with an annulus diameter of 36-55 mm and a right ventricular length > 45 mm.
This is a very promising technology, and I think that it is just a sample of what’s to come in the very near future.
Keywords: Tricuspid regurgitation, Mitral regurgitation, percutaneous prosthetic valve.

For any engineer involved in designing interventional cardiology devices, the goal is to create devices that help solve problems in more complex cases. In the field of coronary intervention, these complex cases may include bifurcations and chronic total occlusion: such lesions are more difficult to treat, mainly because it is more difficult to maintain a good result in the medium- to long-term than with other types of lesion.
Teleflex Medical (USA) has recently launched several guidewires for complex angioplasty. The Bandit guidewire has a low tip load of 0.8 g. It has a long, tapered stainless steel core surrounded by 16 cm of coil; the most distal 10 cm are radiopaque. The distal 17 cm of the guidewire has a polymer jacket. The tip is very slim, measuring 0.008’’. It is available in 200 and 300 cm working lengths.
This guidewire’s lubricity makes it useful for crossing tortuous micro vessels or highly-calcified, sinuous critical lesions.
Normally, Bandit is pre-formed with a slight curve of 30°. It is possible to pre-form a second curve, although I am not a fan of that approach. It is an atraumatic guidewire, similar to the Fielder family (Asahi Intecc, Japan). It could be described as somewhere between the Fielder XT-R (which has 0.6 g tip strength) and the Fielder XT-A (1 g). It is also useful for subintimal advancement, as it can be used with a very small knuckle.
Keywords: Coronary angioplasty wire, complex percutaneous coronary intervention, microcatheters, chronic total occlusion.
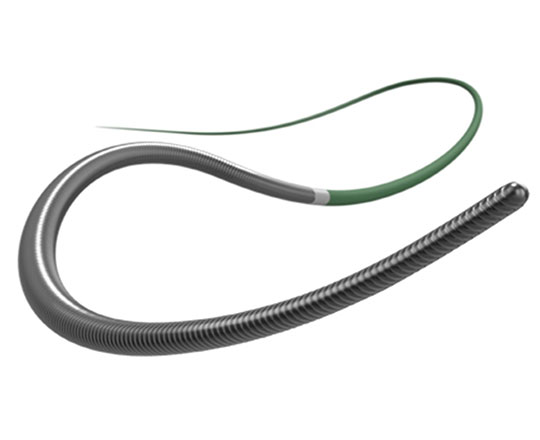
The Raider guidewire is a new coronary angioplasty guidewire for chronic total occlusion, brought to us by Teleflex Medical (USA). Given the fantastic technological advances in recent years, it would seem that continued innovation in guidewire design would be difficult, though not impossible.
This guidewire has a tapered stainless steel core surrounded by a 25 cm coil welded to the core at its proximal end. The distal 30 cm of the guidewire has a polymer coating, the tip is non-tapered, and the tip load is 4 g. It is available in 200 and 300 cm total length.
It is categorized as a medium-support guidewire. Its high flexibility does not limit its use as an added support to facilitate advancing a balloon or stent to more difficult locations.
The design, with a non-tapered tip, means a wider curve of attack can be pre-formed to enable access to more angulated locations. This feature also allows a larger knuckle-type curve to be created if the procedure requires it.
Its penetration power is excellent for increasing the strength of the guidewires used in total coronary occlusions. It is highly suitable for the stick-and-swap technique, in dissection procedures and in Stingray balloon re-entry.
What could it be compared to? A Pitol 200 (Abbott Vascular, USA). What does it add? Superior tactile control.
Keywords: Coronary angioplasty wire, complex percutaneous coronary intervention, microcatheters, chronic total occlusion.
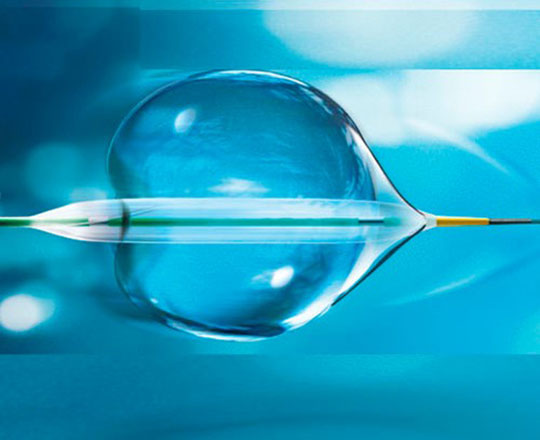
The POT balloon (BrosMed, China) is a dedicated noncompliant postdilatation balloon. Usually, noncompliant postdilatation balloons have shoulders measuring 3.2 mm, the shoulder being the distance from the balloon’s radiopaque marker to its tip. In this balloon, the extra short shoulder, measuring 0.6 mm, allows the device and the operator to remain within the stent, reducing the risk of proximal or distal vessel dissection when the stent is postdilated. It allows controlled postdilatation within the stent.
The balloon, with a diameter of 3 mm, has an entry profile of 0.015 to 0.020 mm and a crossing profile of 0.027 to 0.032 mm. These dimensions are excellent compared to what is already on the market. Worthy of note is the balloon’s low compliance, around 0.1 mm, with a rated burst pressure of 22 atm. The balloon measures between 2.25 and 5 mm in diameter and 6 to 15 mm in length. It is indeed a very useful device for bifurcations.
Keywords: Complex percutaneous coronary intervention, coronary bifurcation, stent postdilation, proximal optimization technique, stent, side-branch, malapposition.
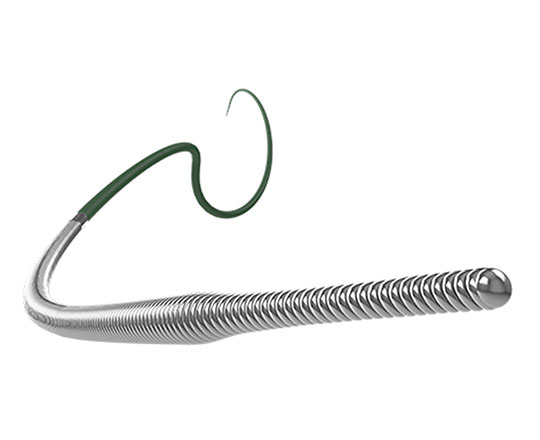
The Warrior guidewire (Teleflex, USA) belongs to the group of high–penetration-power guidewires with a tip load of 14 g. Often, the interventional cardiologist must resort to using peripheral vascular interventional guidewires of 20 g to cross very hard coronary lesions. The Warrior has a long high-support segment designed to facilitate access with long stents (which can be rather inflexible) to difficult locations. It is a slim guidewire with a tapered tip measuring 0.009’’ and micro curves of 2 to 3 mm can be made at its tip (it is not pre-formed). The core is made of stainless steel and the most distal 20 cm is surrounded by coil. This coil has a diameter of 0.014’’ at the most proximal part and 0.009’’ at the most distal part, which provides great stability and capacity for torque response. It has a hydrophilic coating and is available in lengths of 200 and 300 cm.
It is exclusively a penetration guidewire and is used to gain access to highly-calcified proximal or distal capsules and carry out re-entry with R-CART or stick-and-go techniques when a Stingray balloon is used.
One aspect of great practical interest is the following: Teleflex has performed studies on the interaction between tip load and penetration power of their guidewires with different microcatheters, showing that the combination of the Turnpike microcatheter with the Warrior guidewire gives a penetration power of 33.2 g. This means you can avoid resorting to guidewires designed for other vascular territories.
If we had to compare this guidewire to other similar guidewires, we could compare it to the Confianza Pro 12 g (Asahi Intecc, Japan) or the Hornet 14 (Boston Scientific, USA).
Keywords: Coronary angioplasty wire, complex percutaneous coronary intervention, microcatheters, chronic total occlusion.