Over the last decade, understanding of spontaneous coronary artery dissection (SCAD) has progressed from a condition once considered very rare and the subject largely of esoteric case reports to a disease now recognised as a significant cause of acute coronary syndromes, predominantly in young to middle-aged women. Large observational series have been presented, led by groups in the Mayo Clinic, United States and Vancouver, Canada.1-3 This increased understanding has led to the publication of consensus documents on best practice in both the US and Europe.4,5 Despite this, SCAD remains a condition devoid of randomised clinical trial data and debate continues over many aspects of what constitutes optimal management.
In a recent article published in REC: Interventional Cardiology, Bastante et al.6 present data on a highly characterised series from a Spanish centre at the forefront of the progressive management of this condition. Their data provide supporting evidence on findings of direct clinical relevance to contemporary clinical practice and provides novel insight, particularly into the relative merits of antiplatelet therapies for this condition.
The Yip-Saw angiographic classification for SCAD7 coupled with an increased recognition of the central role of intracoronary imaging to aid diagnosis where there is uncertainty, particularly with optical coherence tomography,8 has greatly enhanced the accurate diagnosis of SCAD in the cardiac catheterisation laboratory. In common with other more contemporary and prospective series where there is likely to be less selection bias,2,9 Bastante et al. report SCAD occurring in an older (median age 56), predominantly peri- and post-menopausal female population with a risk factor profile more akin to an age matched general population (48% current or ex-smoker, 36% hypertension, 42% hypercholesterolaemia).6 This further debunks an oft-repeated mantra that SCAD is a disease of pre-menopausal women with few conventional risk factors for ischaemic heart disease. In this and some other studies, only diabetes (6%) seems less prevalent than in the general population. It is interesting to speculate as to whether the adverse vascular effects of diabetes may paradoxically protect against SCAD. Most importantly however, these data remind clinicians not to restrict their consideration of the diagnosis of SCAD to low risk pre-menopausal females.
Accurate diagnosis is the key first management step for SCAD. This paper gives important insights into the diagnostic role of computed tomography coronary angiography (CTCA). It demonstrates that even in a context where the site of SCAD is known from invasive angiography, conventional CTCA missed more than 20% of SCAD locations. This finding confirms that although CTCA is a tempting non-invasive substitute for angiography (particularly given the known increased risk of iatrogenic dissection during invasive angiography in this population10), this approach should not be used for the primary diagnosis of SCAD. The inadequate sensitivity and specificity of CTCA for this diagnosis likely arises because SCAD has a predilection for the mid-distal coronary arteries (76% of cases in this series) where coronary diameters approach the effective spatial resolution of current CTCA technologies. Whether CTCA could still be useful in specific clinical contexts, for example to exclude or to follow progression of high-risk proximal-to-mid vessel dissections, remains to be elucidated.
A key difference between the management of SCAD and atherosclerotic acute coronary syndromes is the increased risk of complications during percutaneous coronary intervention following SCAD. This, coupled with high reported rates of complete healing in conservatively managed SCAD has led to a consensus favouring a non-interventional strategy where possible.4,5 This approach is further supported by the recent demonstration of relatively small infarct sizes in most convalescent SCAD-survivors assessed by cardiac magnetic resonance imaging, with larger infarcts predicted by ST-segment elevation myocardial infarction presentation, reduced TIMI flow and more proximal or extensive dissections.11 In the paper by Bastante et al. 82% of patients were managed conservatively.6 This is in keeping with a large recent prospective Canadian series and suggests that in experienced centres, most SCAD can be managed without percutaneous coronary intervention.2
Optimal medical management following diagnosis remains unclear. This becomes particularly relevant when considering the optimal long-term antiplatelet treatment strategy after SCAD. Antiplatelet therapies have become a mainstay of treatment for atherosclerotic acute coronary syndromes which are characterised by the formation of luminal thrombus on ruptured or eroded atherosclerotic plaque. However, this paper confirms previous findings12 that the burden of true luminal thrombus in SCAD is low, leading the authors to adopt a clinical strategy of early aspirin monotherapy in conservatively managed SCAD (only 7/27 such patients were managed with dual antiplatelet therapy). The longer-term justification for maintenance aspirin has also been questioned as it appears somewhat counter-intuitive to use a medication that prolongs bleeding time as prophylaxis for a condition whose primary pathophysiological event seems to be the development of a spontaneous intramural haematoma. The only potentially informative clinical data come from a single Canadian observational series and found no definitive benefit or harm.1 However, these findings have not yet been validated in other series and whilst this report from Bastante et al.6 will add further fuel to this debate, clinical trial data are urgently needed to address this question.13
The recognition of extra-coronary arteriopathies in SCAD patients14 has led to a consensus favouring arterial screening by brain to pelvis imaging in all SCAD-survivors.4,5 However, it is important to demonstrate that imaging has the potential to alter clinical management or prevent vascular events, especially given the associated X-ray dose if computed tomography arteriography is used.
This is particularly relevant as the most frequent arteriopathy in SCAD survivors is fibromuscular dysplasia which although common, seems of little clinical consequence in most cases. Therefore, the report by Bastante et al.6 of 3 patients in whom intra-cerebral aneurysms identified during screening required closure provides some additional much-needed supportive data that at minimum intracerebral arterial imaging is indeed merited in SCAD-survivors.
Finally and most importantly for patients, Bastante et al. complement data from other series suggesting that although major adverse cardiovascular events (18%) and SCAD recurrence (12%) are significant problems for SCAD-survivors, the disease-related mortality following SCAD is very low.6 Prevention of SCAD recurrence is therefore the key target for clinical trials targeting improved outcomes in SCAD.
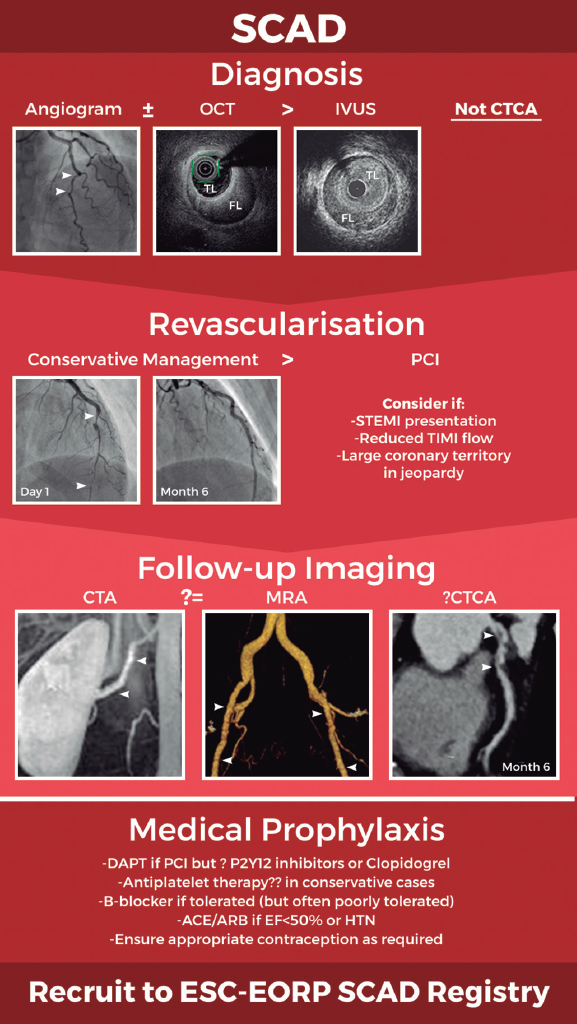
In summary, there has been a paradigm shift in our knowledge of SCAD leading to improved diagnostic accuracy and increasing adoption of a conservative revascularisation strategy (figure 1). However, key questions remain about optimal long-term medical prophylaxis and the best approach to follow-up imaging. International collaboration will be key to generating the data required to definitively address these questions and ensure we continue to improve our approach to the management of this important condition. Recruitment of patients to the new European Society of Cardiology EURObservational Research Programme SCAD registry (CPMS No. 44577, IRAS No. 270314)15 will provide a key platform for future research.
Figure 1. Schematic of current optimal management approach to spontaneous coronary artery dissection (SCAD). Invasive coronary angiography supported by intracoronary imaging if needed remains the diagnostic gold-standard. A conservative approach to revascularisation is favoured except in more extreme presentations. Screening for remote arteriopathies is recommended but the optimal imaging modality and the role of computed tomography coronary angiography remain unclear. Optimal medical management is unknown. All images are from patients from the UK SCAD study (ISRCTN42661582; REC14/EM/0056). ACE/ARB, angiotensin converting enzyme inhibitors or angiotensin receptor antagonists; CTA, computed tomography arteriography; CTCA, computed tomography coronary angiography; DAPT, dual antiplatelet therapy; ESC-EORP, European Society of Cardiology European Observational Research Programme; FL, false lumen; HTN, hypertension; IVUS, intravascular ultrasound; MRA, magnetic resonance angiography; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; TL, true lumen.
CONFLICTS OF INTEREST
D. Adlam has received research funds and in kind support from AstraZeneca, including for spontaneous coronary artery dissection research. He has also received an educational grant from Abbott Vascular to support a clinical research fellow and has conducted consultancy for General Electric, Inc. to support research funds. D. Kotecha has no conflicts of interest to declare.
REFERENCES
1. Saw J, Humphries K, Aymong E, et al. Spontaneous Coronary Artery Dissection:Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol. 2017;70:1148-1158.
2. Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study:in-hospital and 30-day outcomes. Eur Heart J. 2019;40:1188-1197.
3. Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579-588.
4. Adlam D, Alfonso F, Maas A, Vrints C, Writing C. European Society of Cardiology, acute cardiovascular care association, SCAD study group:a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353-3368.
5. Hayes SN, Kim ESH, Saw J, et al.;American Heart Association Council on Peripheral Vascular Disease;Council on Clinical Cardiology;Council on Cardiovascular and Stroke Nursing;Council on Genomic and Precision Medicine;and Stroke Council. Spontaneous Coronary Artery Dissection:Current State of the Science:A Scientific Statement From the American Heart Association. Circulation. 2018;137:e523-e557.
6. Bastante T, García-Guimaraes M, Muñiz M, et al. Contemporary management of spontaneous coronary dissection. REC Interv Cardiol. 2020. https://doi.org/10.24875/RECICE.M20000096.
7. Yip A, Saw J. Spontaneous coronary artery dissection-A review. Cardiovasc Diagn Ther. 2015;5:37-48.
8. Alfonso F, Paulo M, Gonzalo N, Dutary J, Jimenez-Quevedo P, Lennie V, Escaned J, Banuelos C, Hernandez R, Macaya C. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59:1073-1079.
9. Adlam D, Garcia-Guimaraes M, Maas A. Spontaneous coronary artery dissection:no longer a rare disease. Eur Heart J. 2019;40:1198-1201.
10. Prakash R, Starovoytov A, Heydari M, Mancini GB, Saw J. Catheter-Induced Iatrogenic Coronary Artery Dissection in Patients With Spontaneous Coronary Artery Dissection. JACC Cardiovasc Interv. 2016;9:1851-1853.
11. Al-Hussaini A, Abdelaty A, Gulsin GS, et al. Chronic infarct size after spontaneous coronary artery dissection:implications for pathophysiology and clinical management. Eur Heart J. 2020. https://doi.org/10.1093/eurheartj/ehz895.
12. Jackson R, Al-Hussaini A, Joseph S, et al. Spontaneous Coronary Artery Dissection:Pathophysiological Insights From Optical Coherence Tomography. JACC Cardiovasc Imaging. 2019;12:2475-2488.
13. Tweet MS, Olin JW. Insights Into Spontaneous Coronary Artery Dissection:Can Recurrence Be Prevented?J Am Coll Cardiol. 2017;70:1159-1161.
14. Prasad M, Tweet MS, Hayes SN, Leng S, Liang JJ, Eleid MF, Gulati R, Vrtiska TJ. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115:1672-1677.
15. European Society of Cardiology. European Society of Cardiology EURObservational Research Programme SCAD registry. Available at:https://www.escardio.org/Research/Registries-&-surveys/Observational-research-programme/spontaneous-coronary-arterious-dissection-scad-registry. Accessed 8 Jun 2020.
Corresponding author: University of Leicester and NIHR Leicester Biomedical Research Centre, Department of Cardiovascular Sciences, Glenfield Hospital, Groby Road, Leicester LE3 9QP, United Kingdom.
E-mail address: da134@le.ac.uk (D. Adlam).