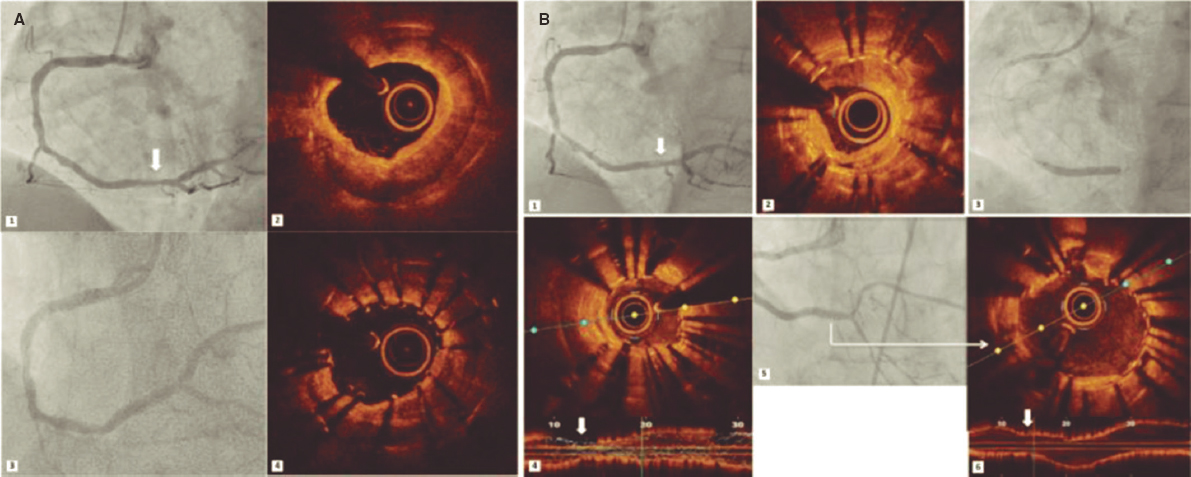
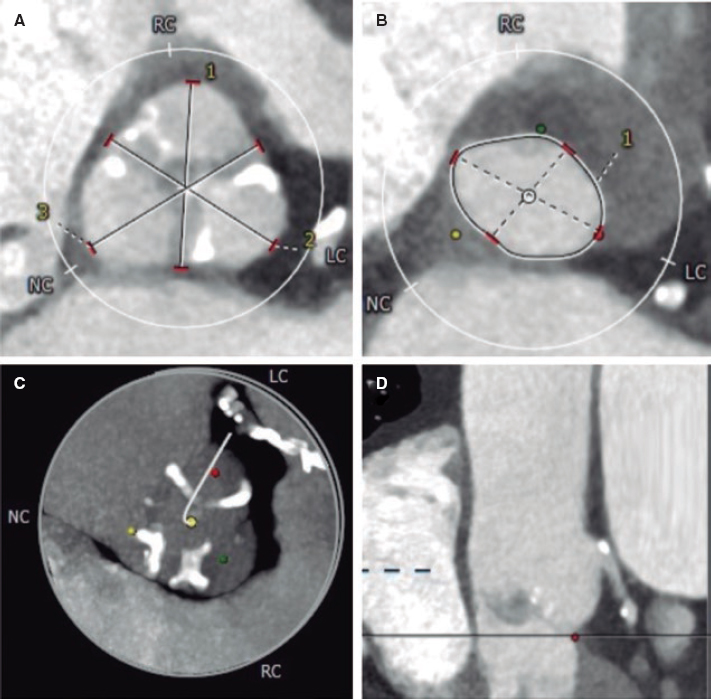
The introduction of endomyocardial biopsy (EMB) for the diagnosis of rejection back in 19721 was considered one of the main advances in the history of heart transplant (HT). Ever since, the EMB has been the gold standard for the diagnosis of rejection. However, this is a repetitive, invasive procedure, often described as uncomfortable by patients and associated with sometimes, serious complications. In this context, in a recent article published on REC: Interventional Cardiology, Tamargo et al.2 shared their combined experience from 2 large national HT centers with the use of EMB via brachial access, a route that is relatively less invasive compared to the femoral access or the most common jugular vein. The authors prove that this is a feasible alternative in 94% of the attempts. Brachial access is safer because it is not associated with some of the major complications of central venous access (mainly pneumothorax and arterial puncture). However, brachial access does not affect the main complication of EMB in the mid-long term: traumatic tricuspid regurgitation occurring after repeated biopsies that can be serious and symptomatic enough to require surgical correction.3 Although brachial access does not reduce procedure time and fluoroscopy time is longer compared to jugular access, it seems evident that this access appears to be more comfortable for patients (although this is based on the testimony of only 19 patients as the authors admit in the limitations section). Therefore, the procedure described is especially appropriate for patients with HT, who represent the largest part of their series and in whom the repeated use of follow-up EMBs is widely accepted as a screening method for graft rejection.
In any case, we should mention that although the EMB still keeps its aura as the gold standard for the diagnosis of rejection, the lack of scientific evidence on this regard is astonishing. In the current era of evidence-based medicine and despite the fact that we have been using this technique for the last 30 years, there is no solid scientific evidence establishing its actual role in the management of patients. In the International Society for Heart and Lung Transplantation clinical practice guidelines,4 systematic EMB for the detection of rejection has a weak recommendation (level IIa) with the lowest possible level of evidence (class C) and with no back up from scientific references.
Also, the histological interpretation of an EMB is rather subjective, the diagnostic criteria have been changed several times, and there is an alarming inter-observer variability.5,6 Actually, its sensitivity and specificity are completely unknown. On the other hand, all HT groups have experienced the frustration of obtaining repeated false positives7 and false negatives8 to the point that there is often little correlation between the anatomopathological degree of rejection and the functional situation of patient and graft.
Therefore, we are not surprised by the numerous attempts to replace EMB with other non-invasive techniques, mainly echocardiographic. Results have been widely variable and they have never been implemented in the real clinical practice.9 Over the last few years, sophisticated techniques like gene expression profiles have been introduced. However, they have only been applied to selected patients (low risk of rejection) and relatively late after the HT (> 2 months to 6 months), which in practice excludes most clinically relevant rejection episodes.10,11
The main problem of these attempts to replace EMB with non-invasive techniques is that we are comparing these new diagnostic approaches to EMB assuming that EMB is the gold standard. However, this argument is wrong from the beginning because this supposed gold standard is not such. To consider the EMB as the gold standard, the current standards of evidence-based medicine should be applied to undisputedly show that the current practice of systematic follow-up with periodic EMBs and histology-based treatment does actually improves clinical results.
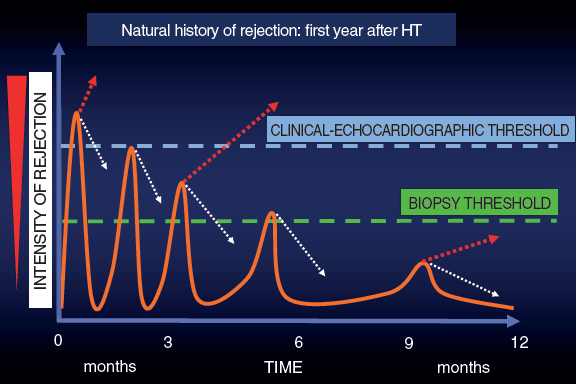
In this context and given the uncertainties due to the frequent false positives and negatives obtained by the EMB, back in the 1990s here at Hospital Universitario Marqués de Valdecilla we developed a periodic clinical-echocardiographic follow-up strategy without performing mandatory EMBs.12 Our strategy is based on this idea: as time goes by after the HT, the episodes of rejection are less aggressive and more spaced-out. Many subside spontaneously, but others may progress until they compromise the function of the graft and are detected either clinically or on the echocardiography. Although we lose diagnostic sensitivity, with this strategy we achieve great clinical specificity because we are only identifying episodes of rejection that do not subside spontaneously (a minority according to experience) and that often respond well to treatment (figure 1).
Figure 1. Graphic representation of the natural evolution of rejection within the first year after a heart transplant. The episodes of rejection are less intense and frequent across time. The dotted white arrows indicate spontaneous resolution of rejection thanks to baseline immunosuppression. The dotted red arrows indicate the continuous evolution of rejection.
Therefore, in these periodic reviews we conduct clinical assessments and obtain follow-up echocardiograms without mandatory EMBs that are only performed in suspected cases. Since we are looking for specificity, we only assess “hard” echocardiographic endpoints (relevant changes in wall thickness or a reduced ejection fraction) suggestive of rejection that warrant treatment. We decide not to use other techniques like strain, which is much more sensitive and would make us lose specificity.
After a 30-year experience and some 600 HTs performed, the use of EMB is marginal in our program.13 The historic mean is 1.24 EMBs per patient, but over the last 10 years it has gone down to only 0.2. In our routine clinical practice we do not perform any EMBs at 1-year follow-up in most patients. Our confidence in this strategy is based on the clinical results that hold a favorable comparison to those of the Spanish Heart Transplant Registry14 both in the mid and long term. If we exclude the first week after the HT (a time when EMBs are rarely performed), the actual survival rate of Hospital Universitario Marqués de Valdecilla is 85% at 1-year follow-up, 75% at 5-year follow-up, and 61% at 10-year follow-up, which is slightly higher compared to that of the Spanish Heart Transplant Registry14 (84% at 1-year follow-up, 73% at 5-year follow-up, and 58% at 10-year follow-up).
In any case, the brachial vein access suggested by Tamargo et al.2 is a warmly welcomed proposal, especially by the patients. Following the classic Stanford Protocol15 a minimum of 14 EMBs should be performed per patient within the first year. This means that the better tolerance shown to this procedure, which is associated with fewer acute complications, can be very relevant for patients given the large number of biopsies they will need to undergo during follow-up.
Several aspects of the current HT management are based on expert opinions and clinical inertia rather than on scientific evidence. Among them, the myth that the EMB is the gold standard should not be accepted given the lack of evidence supporting it. We believe it is important to move forward in this field, but to do so non-invasive alternatives to EMB should change their current approach. Instead of comparing their diagnostic capabilities to those of the EMB (wrong gold standard), they should rather be based on comparing clinical results, which is ultimately what matters. Therefore, we believe that the time has come to design randomized clinical trials comparing clinical results among the different strategies to reduce the use of EMB based on non-invasive methods and review the traditional rule of performing mandatory EMBs.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Caves PK, Stinson EB, Billingham M, Shumway NE. Percutaneous transvenous endomyocardial biopsy in human heart recipients. Experience with a new technique. Ann Thorac Surg. 1973;16:325-336.
2. Tamargo M, Gutiérrez Ibañes E, Oteo Domínguez JF, et al. Endomyocardial biopsy using the brachial venous access route. Description of the technique and 12-year experience at 2 different centers. REC Interv Cardiol. 2020. https://doi.org/10.24875/RECICE.M20000110.
3. Alharethi R, Bader F, Kfoury AG, et al. Tricuspid valve replacement after cardiac transplantation. J Heart Lung Transplant. 2006;25:48.
4. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. Task Force 2:Immunosuppression and Rejection. J Heart Lung Transplant. 2010;29:914-956.
5. Marboe CC, Billingham M, Eisen H, et al. Nodular endocardial infiltrates (Quilty lesions) cause significant variability in diagnosis of ISHLT Grade 2 and 3A rejection in cardiac allograft recipients. J Heart Lung Transplant. 2005;24:S219-S226.
6. Crespo-Leiro MG, Zuckermann A, Bara C, et al. Concordance among pathologists in the second Cardiac Allograft Rejection Gene Expression Observational Study (CARGO II). Transplantation. 2012;94:1172-1177.
7. Winters L, Loh E, Schoen F. Natural History of Focal Moderate Cardiac Allograft Rejection. Is Treatment Warranted?Circulation. 1995;91:1975-1980.
8. Fishbein MC, Kobashigawa J. Biopsy-negative cardiac transplant rejection:etiology, diagnosis and therapy. Curr Opin Cardiol. 2004;19:166-169.
9. Badano LP, Miglioranza MH, Edvardsen T, et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur Heart J Cardiovasc Imaging. 2015;16:919-948.
10. Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890-1900.
11. Crespo-Leiro M, Stypmann J, Schulz U, et al. Clinical usefulness of gene-expression profile to rule out acute rejection after heart transplantation. Eur Heart J. 2016;37:2591-2601.
12. Vázquez de Prada JA. Revisión crítica del papel de la biopsia endomiocárdica en el trasplante cardíaco. Rev Esp Cardiol. 1995;48(Supl 7):86-91.
13. Vázquez de Prada JA, Gonzalez Vilchez F, Rodriguez Entem F, et al. Heart Transplantation without Routine Endomyocardial Biopsies Is Feasible:Experience with a Clinical-Echocardiographic Strategy [abstract]. J Heart Lung Transplant. 2016;35(4S):S32-S33.
14. González-Vílchez F, Almenar-Bonet L, Crespo-Leiro MG, et al. Registro español de Trasplante Cardiaco. XXX Informe oficial de la Sección de Insuficiencia Cardiaca de la SEC (1984-2018). Rev Esp Cardiol. 2019;72:954-962.
15. Billingham ME. Diagnosis of cardiac rejection by endomyocardial biopsy. Heart Transplant.1981;1:25-30.
Corresponding author: Servicio de Cardiología, Hospital Universitario Marqués de Valdecilla, Avda. Valdecilla s/n, 39008 Santander, Cantabria, Spain.
E-mail address: javdpt@gmail.com (J.A. Vázquez de Prada).