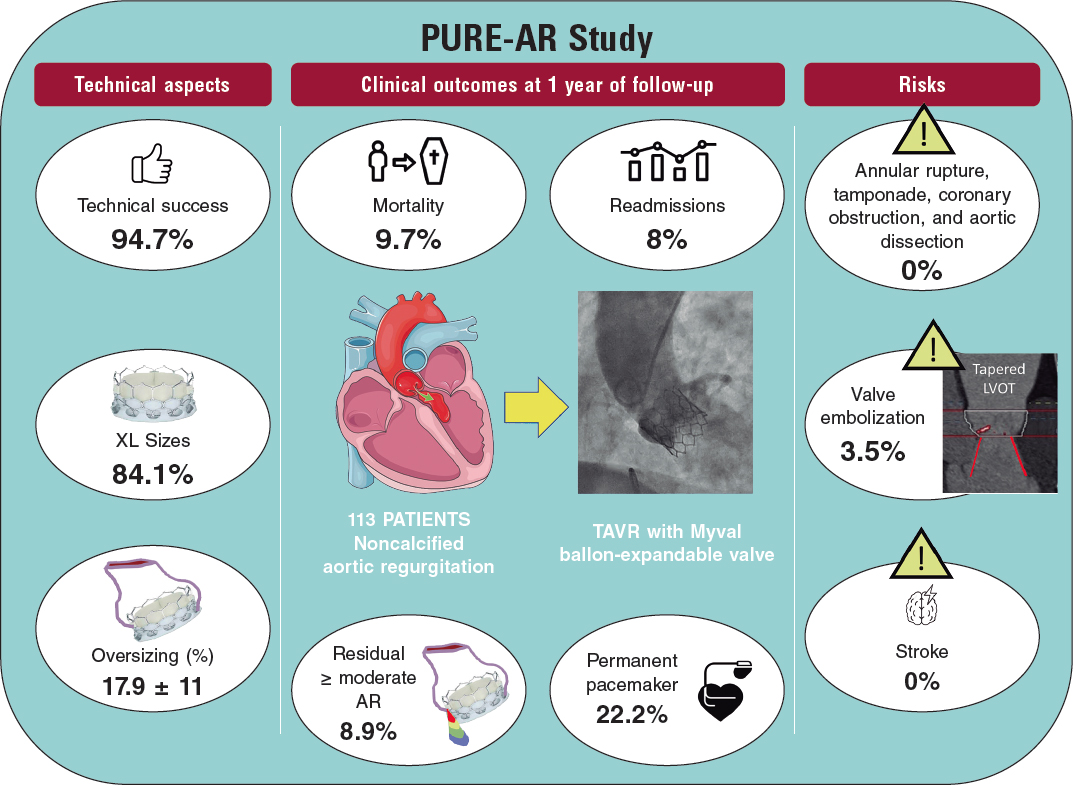
QUESTION: Do you think there is enough evidence to indicate the use of intravascular imaging during percutaneous coronary interventions?
ANSWER: Currently, there is enough evidence on the benefits provided by intracoronary imaging modalities during percutaneous coronary interventions. Actually, the clinical practice guidelines recommend their use. For the most part, evidence has been collected on the use of these imaging modalities to optimize angioplasty. In this sense, the 2018 guidelines on revascularization published by the European Society of Cardiology give a class IIa recommendation and a grade B level of evidence to the use of intravascular ultrasound (IVUS) and optical coherence tomography (OCT) for the optimization of percutaneous coronary interventions (PCI) in selected patients.1 In the case of IVUS, these recommendations are based on multiple studies and meta-analyses that compared the results of angiography vs IVUS-guided PCIs and showed fewer events (including death, infarction or need for new revascularization) with the use of intravascular imaging modalities.2 Guidelines make a specific recommendation on the use of IVUS while performing an angioplasty on the left main coronary artery (class IIa, grade B level of evidence). The other indication for intracoronary imaging modalities established in the guidelines on revascularization is stent failure (class IIa, grade C level of evidence). Several observational studies have proven the utility of IVUS and OCT to detect the causes of thrombosis and restenosis and guide percutaneous treatment.
Q.: Which are the anatomical or clinical contexts with more evidence available?
A.: As I said the clinical context with more evidence available today is the optimization of coronary angioplasty with numerous randomized clinical trials and meta-analyses that confirm the occurrence of fewer events with IVUS-guided PCIs. This effect is especially relevant in the subgroup of patients with complex lesions (including long lesions, bifurcations, and chronic total coronary occlusions) who have a higher risk of events. During the PCI, imaging modalities allow us to determine the size of the stent, optimize its implantation, guarantee its adequate expansion and apposition, and detect possible complications like border dissections.2
The second context with more evidence available (in this case from observational studies) is stent failure. Regarding restenosis, imaging modalities can provide information on its causes (neointimal growth, neoatherosclerosis, underexpansion, disease progression in the stent borders) to determine the most appropriate treatment. Regarding stent thrombosis, intracoronary imaging modalities can detect whether stent thrombosis is due to mechanical causes (like stent underexpansion or incomplete apposition). Also, the OCT allows us to determine whether the cause is associated with an inadequate neointimal coverage of the struts or the rupture of a plaque at the borders or inside the stent.
The anatomical location with highest consensus of all regarding the advantages of using intracoronary imaging modalities (IVUS in particular) is the left main coronary artery. Several studies have proven the utility of this imaging modality to determine the severity of stenosis, the need for revascularization, and eventually to optimize angioplasty.
Q.: Is the IVUS and OCT level of evidence the same?
A.: Since the IVUS has been used much longer than the OCT, there are more clinical studies available on the use of the former, especially as an angioplasty guided imaging modality (to determine the size of the stent and optimize its implantation). However, 2 studies have proven the non-inferiority of OCT vs IVUS for the optimization of stent implantation. The ILUMIEN III study randomized 450 patients who received an OCT, IVUS or angiography-guided angioplasty. It proved the non-inferiority of OCT vs IVUS regarding the minimum stent area obtained. The OPINION study, conducted in Japan, randomized 829 patients to OCT or IVUS-guided stent implantation and proved the non-inferiority of OCT vs IVUS in the primary endpoint of target vessel failure including cardiac death, treated vessel related infarction or new revascularization on the lesion operated on. This prompted that the latest iteration of the revascularization guidelines published by the European Society of Cardiology to give the same class of recommendation (IIa) to the use of IVUS and OCT for PCI optimization.
Q.: What are the advantages of OCT with respect to IVUS during percutaneous coronary interventions?
A.: The OCT has 10 times more resolution than the IVUS, which allows much more detailed visualizations of the arterial wall and its interaction with the stent. This makes it much more sensitive to detect phenomena like stent incomplete apposition or stent border dissection. In the long-term assessment of the stent it also allows us to study the vessel tissue repair and determine whether the stent is completely covered by tissue. Regarding acute coronary syndromes, the OCT is much more sensitive to detect the presence of thrombi and it can characterize the underlying cause much better. In this sense, the OCT allows us to distinguish in vivo acute coronary syndromes due to the rupture of the plaque from those due to erosions, calcified nodules or non-atherosclerotic causes (such as spontaneous coronary artery dissections or embolizations). This is relevant to guide treatment because it can determine whether it is necessary to operate or just use conservative strategy.3
Also, the OCT offers several advantages in the assessment of the causes of stent failure. Regarding in-stent restenosis, it allows us to detect its underlying mechanisms like underexpansion, neointimal tissue growth or disease progressions. Also, the high resolution of this imaging modality has enabled the in vivo detection of neoatherosclerosis as a common cause for restenosis. The analysis of these mechanisms is essential to determine the best therapeutic strategy. For example, in the case of disease progression in the borders of the stent it will be necessary to implant a new stent. However, in cases of stent underexpansion it will be necessary to expand the stent with high pressure dilatations, in some cases, even use plaque modification techniques. If the cause of restenosis is neointimal growth a new stent or a drug-coated balloon can be implanted. Regarding neoatherosclerosis, at the moment there is not enough evidence to determine whether the best strategy is to implant a new stent or use a drug-coated balloon. Still, some data available suggest that both options may be useful.
Regarding stent thrombosis, the OCT allows us to demonstrate in vivo that this is often a multifactor phenomenon and that the cause is not only the lack of stent coverage (as it was initially thought with drug-eluting stents). Instead, it can be due to other factors like neoatherosclerosis with plaques ruptured inside the stent or around its borders, stent underexpansion, incomplete apposition or restenosis. Again, this is an important piece of information to guide the interventional treatment and correct the underlying cause.
Q.: Which cases would be eligible for using IVUS and which for using OCT?
A.: There are 2 anatomical locations where the IVUS is superior compared to the OCT: 1) ostial lesions, due to the impossibility to clean out the blood from the vessel to acquire good images on the OCT; and 2) the left main coronary artery, especially when the ostial segment is involved. Before, the size of the left main coronary artery was a limitation when performing OCT, but with the systems we have today we can see almost all left main coronary arteries unless they are too big. Several studies show the utility of IVUS to assess the severity of the left main coronary artery and for guidance purposes during the angioplasty. At this moment, several studies in the pipeline are assessing the use of OCT while performing angioplasties on the distal left main coronary artery. Probably in the mid and distal left main coronary artery setting, the OCT will be as useful as the IVUS. Maybe even more in the assessment of bifurcations. However, if the ostial segment is involved and we want to see it, we better use the IVUS. Another situation where the IVUS may be preferred over the OCT is to see kidney damage given the need to use contrast for the acquisition of OCT images. In this sense, I should say that the use of contrast can be optimized when performing OCT-guided angioplasties to avoid unnecessary angiographies. A single injection of contrast allows us to perform an angiography and an OCT at the same time. With a single OCT-pullback the reference areas can be selected, and the diameter and length of the vessel can be obtained avoiding the need to perform multiple angiographies.
The OCT is superior to the IVUS and should be the imaging modality of choice to assess stent failure (thrombosis and restenosis) because it is much more precise to determine the underlying mechanism. It is also superior for the assessment of acute coronary syndromes because it is much more sensitive to detect thrombi and distinguish acute coronary syndromes due to plaque rupture from those due to other mechanisms like erosions or non-atherosclerotic causes. The OCT is superior to the IVUS for the assessment of bifurcations because it allows online 3D reconstructions that provide relevant information on the anatomy of the bifurcation and can optimize the angioplasty. An important advantage of the OCT over the IVUS conventional systems is the possibility of a simultaneous registry with an angiography incorporated to the system that does not require the use of any additional software. This facilitates significantly the use of OCT to guide and optimize stent implantation because it offers an online co-registration of the angiographic location of each and every one of the images seen on the OCT.
Q.: What studies are necessary to establish the role of these imaging modalities during percutaneous coronary interventions?
A.: Regarding IVUS, several studies show that its use during the angioplasty can improve the prognosis of patients by reducing the occurrence of events. Regarding the OCT, the primary endpoint of the ILUMIEN IV study, currently in the pipeline, is to show that OCT-guided PCIs can improve stent implantation and reduce clinical events compared to angiography-guided PCIs only. The positive effects of using intracoronary imaging modalities are especially relevant in the subgroup of lesions with higher risk of failure (including long lesions, bifurcations, restenosis, and chronic coronary occlusions). Actually, it is in these patients in whom we should encourage the use of IVUS or OCT.
Beyond the evidence generated in the clinical trials, in order to promote the use of intracoronary imaging modalities, interventional cardiologists need to be trained on how to interpret them. At the same time, imaging systems need to improve to make them easier to use during the procedures. For example, the use of fast systems of image acquisition integrated in the cath lab that allow the operator to use the controls from the table, and co-registration of angiography are some of the tools that can improve the use of these imaging modalities.
Q.: What technical advances are available today or could be available in the near future regarding the OCT?
A.: Among the technical advances currently under research, the most relevant ones have to do with the possibility of estimating and assessing the physiological parameters from OCT acquired 3D reconstructions. This would allow us to use 1 imaging modality only (the OCT) to determine the need to treat and optimize the intervention.
Plaque characterization (especially calcium) using dedicated software is another important field of study. Ultrafast pullbacks will allow us to reduce the amount of contrast needed, and the combination of IVUS plus OCT in the same catheter are other advances being made today.
REFERENCES
1. Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87?165.
2. Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1:guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281-3300.
3. Johnson TW, Räber L, Di Mario C, et al. Clinical use of intracoronary imaging. Part 2:acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making:an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2019;15:434-451.
Corresponding author: Unidad de Cardiología Intervencionista, Hospital Clínico San Carlos, Martín Lagos s/n, 28040 Madrid, España.
E-mail address: nieves_gonzalo@yahoo.es (N. Gonzalo).