ABSTRACT
Primary angioplasty is now clearly established as the best reperfusion strategy for patients with ST-segment elevation myocardial infarction (STEMI), but the best strategy for significant stenosis at non-culprit vessels has not been adequately studied. Several randomized trials have been previously performed, but all of them with soft primary endpoints and consequently a low number of patients. The COMPLETE trial, for the first time, provides us with solid scientific evidence about what we should do in patients with STEMI and multi-vessel disease. This study included more than 4000 patients and has shown that complete revascularization reduces significantly the risk of cardiovascular death or myocardial infarction.
Keywords: STEMI. Percutaneous coronary revascularization. COMPLETE trial.
RESUMEN
La angioplastia primaria está reconocida como la mejor estrategia de reperfusión en el infarto de miocardio con elevación del segmento ST. No obstante, la mejor estrategia para el tratamiento de las lesiones coronarias significativas en arterias no relacionadas con el infarto no se había estudiado convenientemente. Hasta la fecha se habían realizado varios estudios aleatorizados pero con objetivos de beneficio clínico de gravedad menor o «blandos» y pocos pacientes. Por primera vez, el estudio COMPLETE proporciona evidencia científica sólida sobre la estrategia terapéutica en pacientes con infarto de miocardio con elevación del segmento ST y enfermedad multivaso. Este estudio, que incluyó a más de 4.000 pacientes, ha demostrado que la revascularización completa reduce significativamente el riesgo combinado de mortalidad o infarto de miocardio.
Palabras clave: IAMCEST. Revascularización coronaria percutánea. Estudio COMPLETE.
Abreviaturas: STEMI: ST-segment elevation myocardial infarction.
The solid results of the recently published COMPLETE trial1 clearly show that the revascularization of nonculprit lesions after successful primary percutaneous coronary intervention (PCI) for the management of ST-segment elevation myocardial infarction (STEMI) improves long-term outcomes. This is evident today, will probably be accepted as a general strategy, and is one of the last steps in a long journey (figure 1). It all started a long time ago when a gradually better and more aggressive management of acute myocardial infarction improved short and long-term prognosis, and reduced mortality rates to unimaginable levels only a few years ago. Once upon a time, and based on anatomopathological findings, there was this wrong assumption that coronary artery occlusions during acute myocardial infarction were the consequence of myocardial necrosis and not the other way around. Only in the 1980s it became clear that opening the culprit, occluded coronary artery improved the outcomes. Immediate revascularization procedures played a significant role together with better medical therapies, reorganizing the existing strategies, and much better secondary prevention measures immediately after an acute event.2-4
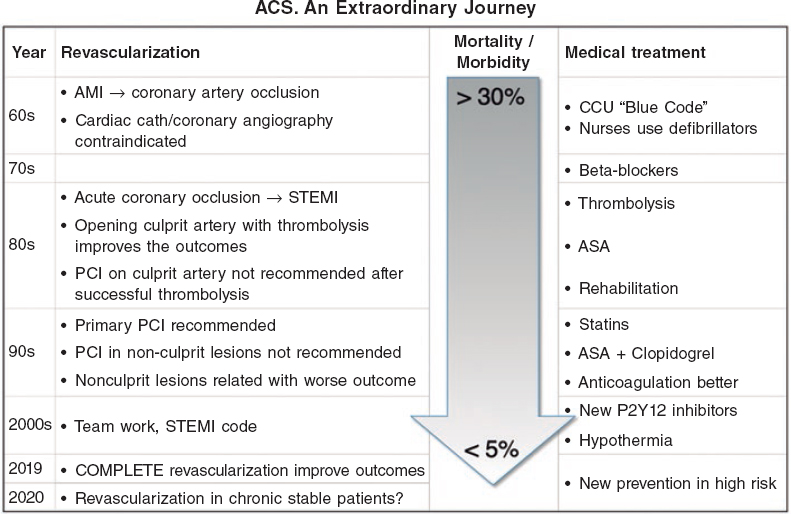
Figure 1. Evolution of revascularization, medical therapies, and outcomes in the management of STEMI. ACS, acute coronary syndrome; AMI, acute myocardial infarction; ASA, acetylsalicylic acid; CCU, coronary care unit; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
To this day, all advances made on revascularization were associated with the culprit vessel (the immediate, successful and sustainable recanalization of the culprit vessel), yet the efforts made with diseased non-culprit vessels have yielded unclear results.
The COMPLETE trial compared a strategy of culprit lesion only versus complete revascularization in patients with acute STEMI with other significant coronary artery stenosis identified at the time of the primary PCI. The study included 4041 patients with a median follow-up of 3 years. Significant reductions in the first coprimary endpoint of cardiovascular death or myocardial infarction and second coprimary endpoint of cardiovascular death, myocardial infarction or ischemia-driven revascularization were seen in the complete revascularization arm compared to the group where only on the culprit artery was revascularized. The benefit favoring complete revascularization was observed early after inclusion in the trial and became more evident through the 3-year follow-up (figure 2). These observations were consistent through the different subgroups. There was a small, nonsignificant increase in major bleeding and contrast-induced acute kidney injury. The benefit was mainly driven by significant reductions in myocardial infarction and ischemia-driven revascularization.
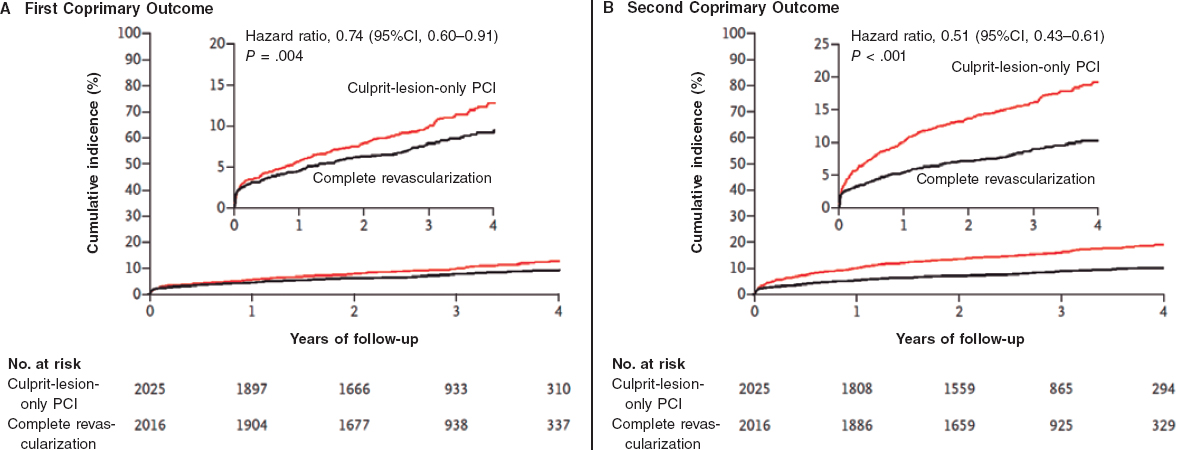
Figure 2. COMPLETE trial main results. Kaplan-Meier estimates for the first coprimary outcome (cardiovascular mortality or new myocardial infarction) and second coprimary outcome (cardiovascular mortality, new myocardial infarction, or ischemia-driven revascularization). CI, confidence interval; PCI, percutaneous coronary intervention. Reproduced from Mehta et al.1 with permission.
Previous studies with soft primary endpoints failed to show that complete revascularization could be beneficial in terms of death or myocardial infarction, but the number of patients included in these trials was very small,5-10 ranging from 69 patients in the HELP-AMI trial6 to 885 patients in the COMPARE-ACUTE.8 These trials combined represent just a small fraction of the number of patients included in the COMPLETE trial.
As most trials, the COMPLETE also raises some practical questions. First, is the benefit clinically significant? The COMPLETE trial showed robust results but failed to show reductions of cardiovascular mortality, heart failure and all-cause mortality. It is well accepted that multivessel disease is a clear risk factor for mortality after STEMI11; however, the inclusion and specially exclusion criteria of the COMPLETE trial selected a group of low risk patients. Cardiovascular mortality was only 1% per year and it is almost impossible to show mortality reductions in this population. But there was a significant reduction in the rate of myocardial infarction (2.8% vs 1.9% per year), unstable angina (2.2% vs 1.2% per year), and ischemia-driven revascularization (2.8% vs 0.5% per year) without an excess of major complications. This will make most interventional and clinical cardiologists take this strategy seriously. Although the recommendations established by the guidelines on secondary prevention were followed in the trial, secondary prevention is evolving very fast,12 complementing revascularization strategies, but also decreasing the relative role of each component.
Second, is routine complete revascularization for all? Actually it is in all the cases that meet the COMPLETE inclusion and exclusion criteria (table 1), but not in patients with small vessel disease, non-significant epicardial coronary artery stenosis, prior bypass surgery, and others. We should not forget that the COMPLETE trial excluded patients with cardiogenic shock, and routine complete revascularization has proven harmful in these patients.13 The question of whether all patients that meet the COMPLETE criteria should undergo complete revascularization is more difficult to answer. Identifying patients at higher risk, based on clinical characteristics, and additional information on coronary anatomy and in particular on plaque stability is indicative of the target population that may benefit the most. This information may be obtained from the trial database.
Table 1. Eligibility of patients in the COMPLETE trial. Major inclusion and exclusion criteria
| Inclusion criteria |
|---|
| STEMI |
| Successful PCI on the culprit lesion |
| At least 1 non-culprit lesion |
| Non-culprit lesion diameter ≥ 2.5 mm |
| Non-culprit lesion stenosis > 70% or |
| 50%–60% stenosis and FFR ≤ 0.8 |
| Immediate revascularization within 72 h following the index PCI |
| Exclusion criteria |
| Planned surgical revascularization |
| Prior bypass surgery |
FFR, fractional flow reserve; PCI, percutaneous coronary revascularization; STEMI, ST-segment elevation myocardial infarction. |
Third, a key question related to the timing of complete revascularization. The COMPLETE trial recommended a staged rather than a single revascularization procedure. Other trials have assessed complete revascularizations in a single procedure in patients with STEMI and multivessel disease with good safety and efficacy data.7,10 Complete revascularizations in a single procedure reduced cardiovascular events compared to staged-revascularizations in patients with NSTEMI.14 Currently, the BioVasc trial (NCT03621501) is comparing complete revascularizations in single procedures to staged procedures in patients with STEMI and NSTEMI. According to the COMPLETE trial, complete revascularization should be performed during index hospitalizations, but it did not show data on single-stage revascularizations.
Finally, could revascularization improve the outcomes of stable coronary disease? Actually this is out of the scope of the COMPLETE trial, and so far the evidence available today is very weak, with only marginal benefits for revascularization, if any.15 The ongoing ISCHEMIA trial16 is comparing the benefit of revascularization plus medical therapy versus medical therapy alone in over 5000 patients and its results will be published very soon. Obviously, the results will be crucial to improve the invasive strategy for the management of coronary heart disease. Would complete revascularization be beneficial in patients with non-ST segment elevation acute coronary syndromes? Probably, but we do not know it yet and there are no studies exploring this hypothesis.
Meanwhile, please go ahead and check if STEMI patients with prior successful primary PCIs qualify as eligible candidates for COMPLETE revascularization within 72 hours (before hospital discharge).
CONFLICTS OF INTEREST
J. López-Sendón reported having received grants from McMaster University (Hamilton, Ontario, Canada) while conducting this study. J. López-Sendón and R. Moreno are coauthors of the article reviewed on this paper. R. Moreno is Associate Editor of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
REFERENCES
1. Mehta SR, Wood, DA. Storey RF, et al.;for the COMPLETE Trial Steering Committee and Investigators. Complete revascularization with multivessel PCI for myocardial Infarction. N Engl J Med. 2019. https://www.nejm.org/doi/10.1056/NEJMoa1907775.
2. González-Juanatey JR, Agra Bermejo R, López-Sendón J. Una historia resumida. Impacto de los avances en cardiopatía isquémica. Rev Esp Cardiol. 2017;17(Suppl A):2-6.
3. Ibañez B, James S, Agewall S, et al.;, for the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018:39:119-177.
4. Neumann FJ, Sousa-Uva M, Ahlsson A, et al.;for the The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
5. Moreno R, Mehta SR. Nonculprit Vessel Intervention:Let's COMPLETE the Evidence. Rev Esp Cardiol. 2017;70:418–420.
6. Di Mario C, Sansa M, Airoldi F, et al. Single vs multivessel treatment during primary angioplasty:results of the multicenter randomized HEpacoat for cuLPrit or multi vessel stenting for Acute Myocardial Infarction (HELP-AMI) study. Int J Cardiovasc Intervent. 2004;6:128-133.
7. ald DS, Morris JK, Wald NJ, et al. PRAMI Investigators. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369: 1115-1123.
8. Engstrøm T, Kelbæk H, Helqvist S, et al.;for the DANAMI-3—PRIMULTI Investigators. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI):an open-label, randomised controlled trial. Lancet. 2015;386:665-671.
9. Smits PC, Abdel-Wahab M, et al. for the Compare-Acute Investigators. Fractional Flow Reserve–Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376:1234-1244.
10. Politi L, Sgura F, Rossi R, et al. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction:Major adverse cardiac events during long-term follow-up. Heart. 2010;96:662-667.
11. Moreno R, García E, Elízaga J, et al. Results of primary angioplasty in patients with multivessel disease. Rev Esp Cardiol. 1998;51:547-555.
12. Knuuti J, Wijns W, SarasteA, et al.;for the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes:The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2019. https://doi.org/10.1093/eurheartj/ehz425.
13. Thiele H, Akin I, Sandri M, et al.;CULPRIT-SHOCK Investigators. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med. 2017;377:2419-2432.
14. Sardella G, Lucisano L, Garbo R, et al. Single-staged compared with multi-staged PCI in multivessel NSTEMI patients:the SMILE trial. J Am Coll Cardiol. 2016;67:264-272.
15. Stergiuopoulos K, Boden WE, Hartinhan P, et al. Percutaneous Coronary Intervention Outcomes in Patients With Stable Obstructive Coronary Artery Disease and Myocardial IschemiaA Collaborative Meta-analysis of Contemporary Randomized Clinical Trials. JAMA Intern Med. 2014;174: 232-240.
16. Hochman JS, Reynolds HR, Bangalore S, et al.;for the ISCHEMIA Research Group. Baseline characteristics and risk profiles of participants in the ISCHEMIA randomized clinical trial. JAMA Cardiol. 2019;4:273-286.
Corresponding author: Servicio de Cardiología, Hospital Universitario La Paz, P.º de la Castellana 261, 28046 Madrid, Spain.
E-mail address: jlopezsendon@gmail.com (J. López-Sendón).











