Case Resolution
With an 8.5% score in the Society of Thoracic Surgeons risk score for mortality rate, we decided to implant one Evolut PRO transfemoral bioprostheis on an underexpanded bioprosthesis. The computed tomography confirmed the lack of coverage of the bioprosthetic stent over the aortic annulus at the level of the Valsava, non-coronary and right coronary sinuses (figure 1), indicative of stent recoil of the stent harboring the bioprosthetic leaflets as the possible mechanism of periprosthetic failure and causing malapposition with the aortic annulus. The perimeter of the aortic annulus was 79.3 mm (minimum diameter: 22 mm; maximum diameter: 25 mm).
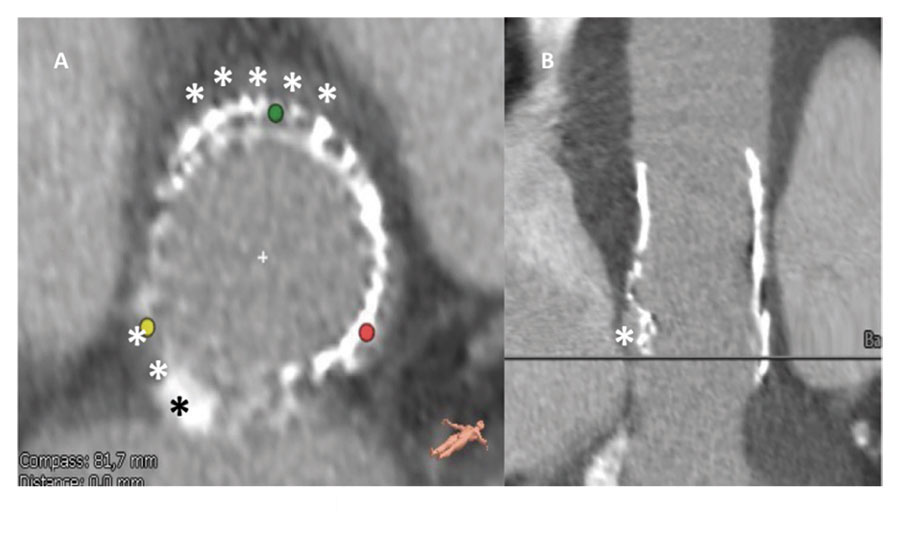
Figure 1. Lack of coverage of the bioprosthetic stent over the aortic annulus at the level of the Valsava, non-coronary and right coronary sinuses (asterisk). The colored dots are indicative of the location of coronary sinuses.
One self-expandable prosthesis was selected with leaflets at the supra-annular level, since it has already been confirmed that in valve-in-valve procedures, the hemodynamic outcome is better compared to the annular implantation that leaves a more significant trans-prosthetic gradient. Also, the position of the prosthesis inside the prosthesis needs to be optimal, which makes the Evolut R the perfect device for this kind of procedure for its recapture and repositioning capabilities.
The selection of the size of the prosthesis to be implanted was based on the true internal diameter of the 25 mm Perceval L valve, that is, 21.5 mm to 23 mm according to the manufacturer. The size recommended for the Evolut PRO is between 26 mm and 29 mm. Finally, the 29 mm size was picked to ensure a correct annular sealing.
The procedure was conducted under general anesthesia, with mechanical ventilation and transesophageal echocardiography (figure 2).
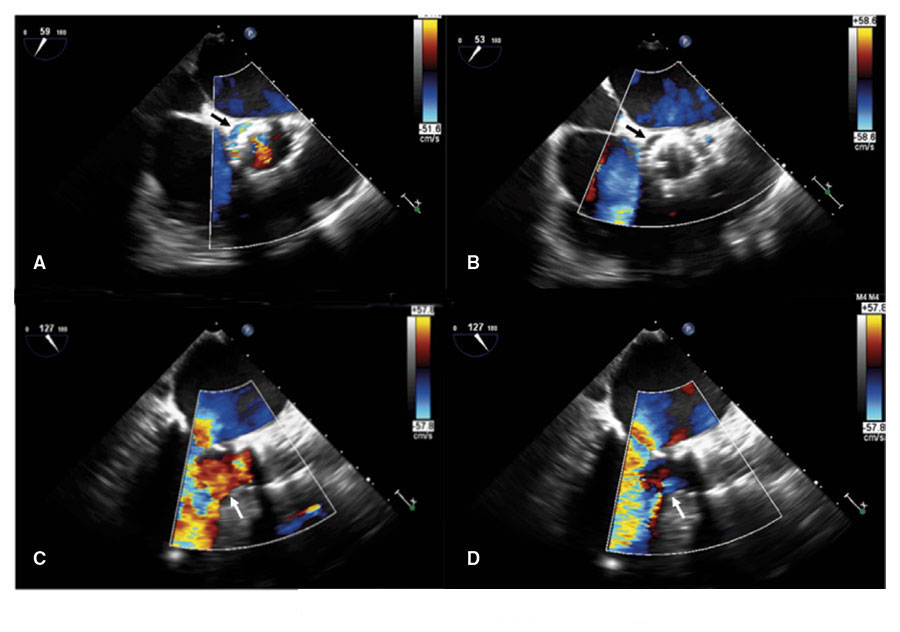
Figure 2. Transesophageal echocardiography: transversal view of the aortic annulus (A, B) and three chambers (C, D). A, C: regurgitation jet towards the left ventricle before the percutaneous implant of the aortic valve (arrows). B, D: no aortic regurgitation jet after the percutaneous implant of the aortic valve (arrows).
We picked the working projection where the inferior edge of the malfunctioning prosthesis was aligned. The pigtail catheter was placed proximal to the dysfunctional prosthesis to conduct the corresponding maneuvers during the implantation stage. The radio-opacity of the Perceval prosthesis gives us enough information for the correct deployment of the Evolut device.
The procedure was conducted using fluoroscopic monitoring and transesophageal echocardiography. The Perceval prosthetic leaflets were placed intra-annularly and for correct implantation purposes, the location picked for the Evolut device was the inferior edge of the Perceval prosthesis in such a way that the inferior edge of the Evolut 2 mm would match the Perceval device underneath. The deployment of the Evolut device is slightly distal to the dysfunctional bioprosthesis (figure 3). When 80% of the deployment had already been completed, the transesophageal echocardiography (figure 2) confirmed the correct positioning and functioning of the Evolve device, and the aortic failure being sealed. The remaining of the prosthesis was then fully deployed. The procedure was conducted without any conduction alterations and the it was completed uneventfully. The progression of the patient was good with no traces of heart failure.
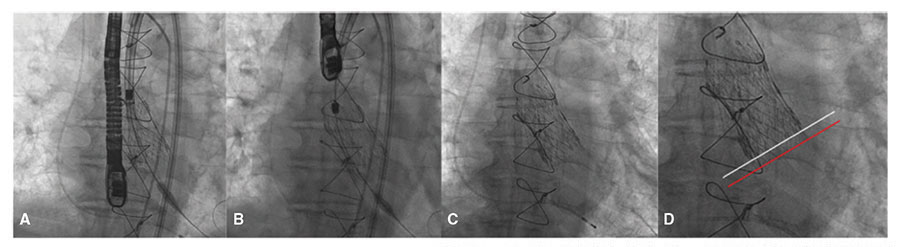
Figure 3. Implant of Evolut PRO 29 over Perceval L. A: deployment of the prosthesis at 80%. B: prosthesis being deployed in its final position. C: removal of the release catheter. D: inferior edge of the Perceval prosthesis (white line) and inferior edge of the Evolut PRO (red line).
CONFLICTS OF INTEREST
R. Trillo Nouche is proctor for Medtronic.
Corresponding author: Servicio de Cardiología, Complejo Hospitalario Universitario de Santiago de Compostela, Rúa da Choupana s/n, 15706 Santiago de Compostela, A Coruña, Spain.
E-mail address: ramirotrillo@mac.com (R. Trillo Nouche).

